Novel polyoxometallate compound, preparation method thereof and application thereof
A technology of oxo-salt compounds and multi-metals, applied in metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem of low photocatalytic efficiency, inability to fully utilize, and Mineralization of organic matter and other issues, to achieve the effect of simple preparation method, good decolorization and degradation ability, and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] ①Dissolve 18.2g of sodium tungstate in 100ml of deionized water, and adjust the pH value of the system to 6-7 with glacial acetic acid to obtain solution A;
[0038] ② 1.61g, ZrOCl 2 Dissolve in 10ml deionized water to obtain solution B;
[0039] ③Add solution B to solution A, stir evenly, adjust the pH value of the system to 4.5-5.5 with glacial acetic acid, and then reflux at 70°C for 15-30 minutes;
[0040] ④ Dissolve 1.075g of stannous sulfate in 10ml of deionized water, and adjust the pH value of the system to 5-5.5 with 0.2g / ml sodium acetate aqueous solution to obtain solution C;
[0041] ⑤Under nitrogen protection conditions, add 30ml of solution C to step ③, raise the temperature to 95°C, reflux for 1.5h, add 20ml, 0.25g / ml KCl aqueous solution 10min before the end of the reaction;
[0042] ⑥ Cool the solution to room temperature after the reaction, remove insoluble matter by filtration, add 100-150ml of absolute ethanol to the above solution, stir slowly, af...
Embodiment 2
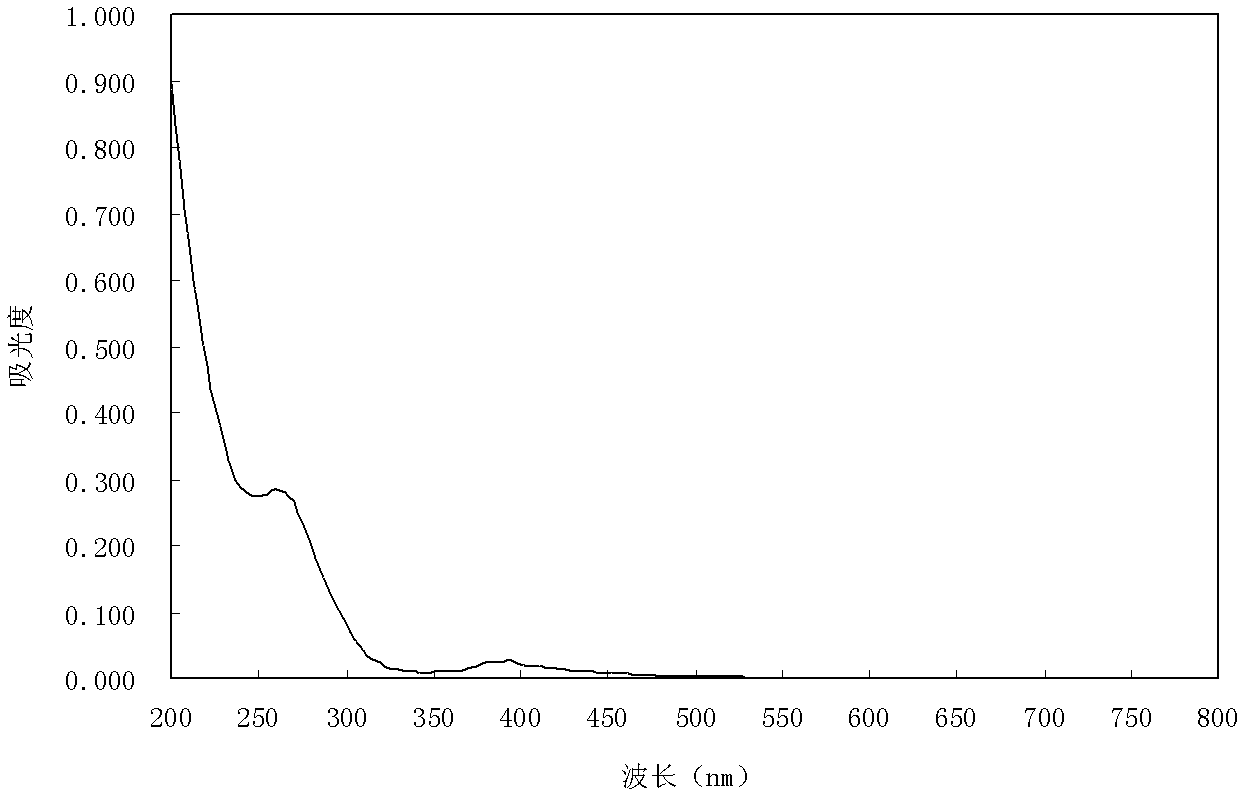
[0051] Take 20ml, 5mg / L Acid Scarlet 3R aqueous solution in a beaker, add 20mgK 6 wxya 11 o 39 sn II 12H 2 O catalyst (the concentration of the catalyst in the solution: 1g / L), placed in the outdoor sunlight for 5 hours, with an average temperature of 20°C. After photocatalytic degradation for 5 hours, the decolorization rate of Acid Scarlet 3R aqueous solution was 65%. Figure 6 It is the ultraviolet-visible spectrogram before (a) and after (b) of the photolysis of this embodiment. It can be seen from the figure that the absorption peak of Acid Scarlet 3R aqueous solution in the visible region is significantly reduced, indicating that its molecular azo bond has been destroyed. , and the solution after photolysis has a large absorption in the ultraviolet region, indicating that a new intermediate product is generated.
Embodiment 3
[0053] For solution initial pH=4, 60ml, 15mg / L methyl red solution, add 15mgK 6 wxya 11 o 39 5n II 12H 2 O catalyst (the concentration of the catalyst in the solution: 0.25g / L), placed in the outdoor sunlight for 1h, the decolorization rate reached 96.9%, most of the azo group of methyl red has been destroyed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com