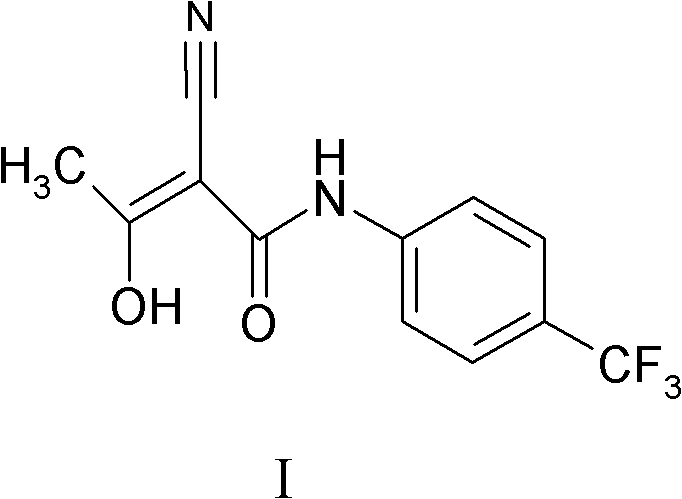
Use of the combination of teriflunomide and glatiramer acetate for treating multiple sclerosis
A technology of glatiramer acetate and multiple sclerosis, which is applied in the direction of drug combinations, medical preparations containing active ingredients, organic active ingredients, etc., and can solve the undisclosed problems of effectiveness and safety in the treatment of multiple sclerosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
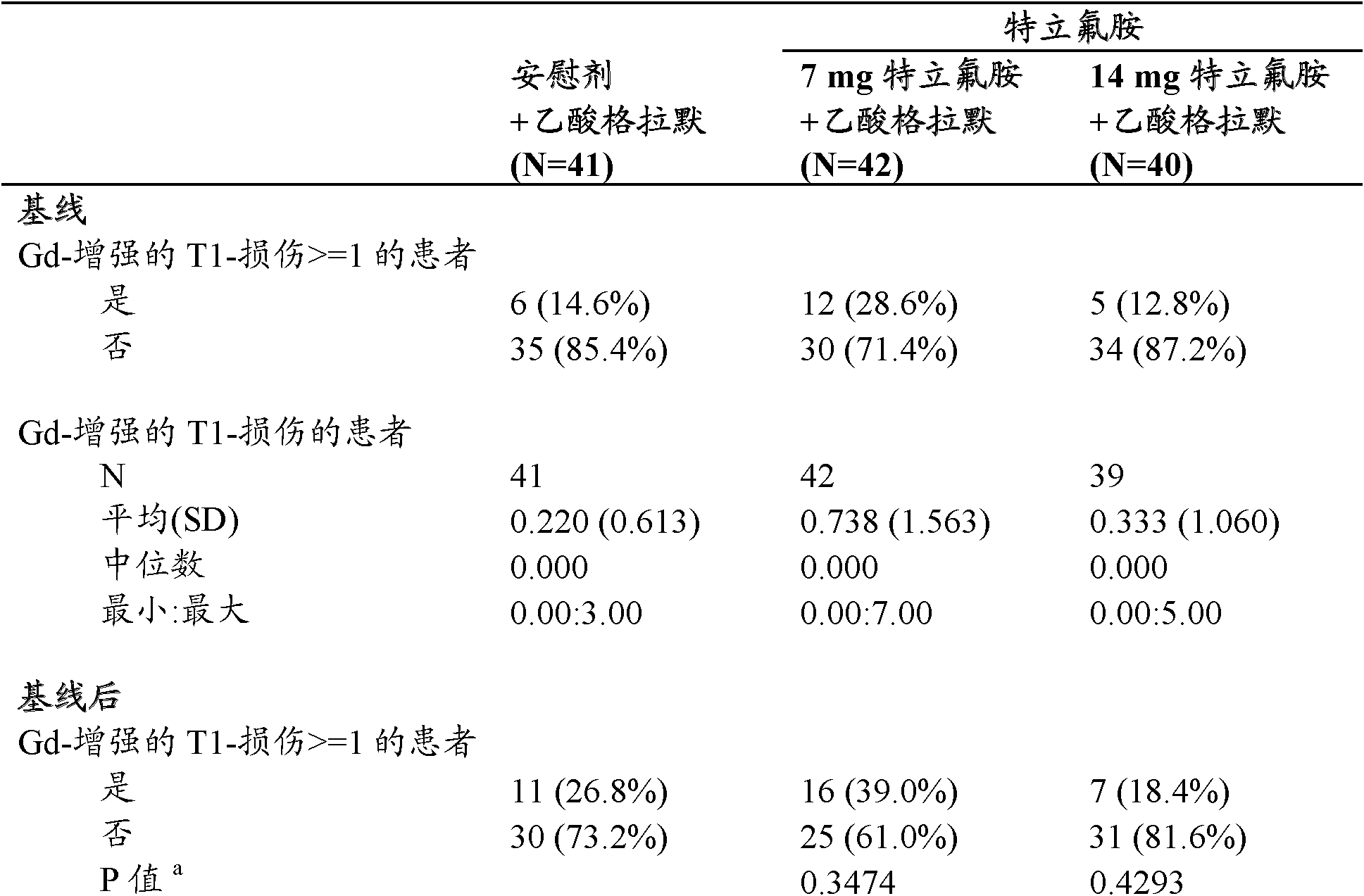
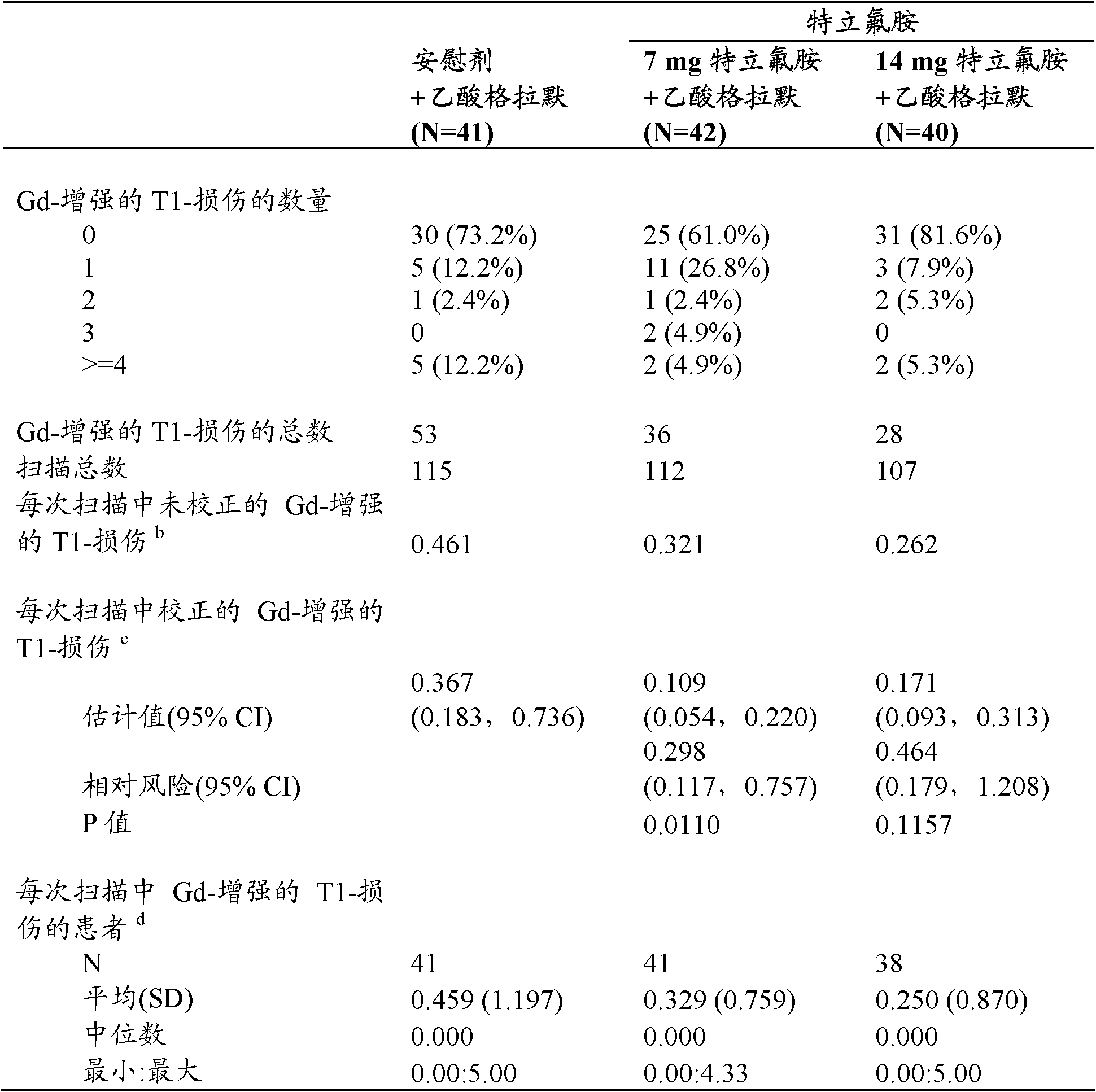
[0077] A placebo-controlled, randomized, double-blind study in relapsing-remitting multiple sclerosis patients coadministered with stable doses of glatiramer acetate. The subcutaneous dose of glatiramer acetate is 20 mg per day.
[0078] Approximately 40 patients were treated in each treatment group (placebo group: 41, 7 mg group: 42, and 14 mg group: 40). Demographic and baseline disease characteristics were essentially comparable among the 3 treatment groups. The mean age of the study population was 41.4 years. The majority of patients were women (78.9%) with relapsing-remitting MS (94.3%) who had been diagnosed with MS since about 8 years ago and about 41% of patients had no relapse in the previous year. Baseline Expanded Disability Status Scale (EDSS) scores were similar between treatment groups (approximately 2.5). 28.6% of patients in the 7 mg group had magnetic resonance imaging (MRI) disease activity (at least one T1-Gd lesion) at baseline, slightly more than the pl...
Embodiment 2
[0114] Design / Methods: Of the 123 patients randomized to study treatment during the first 6 months of Example 1 (placebo: 40; 7 mg: 42; 14 mg: 41), 96 completed The 6-month treatment period and agreed to continue dosing for another 6 months (placebo group: 37, 7 mg group: 30, 14 mg group: 29). Of the 96 treated patients, 5 patients withdrew from the study treatment prematurely (placebo group: 3, 7 mg group: 0, 14 mg group: 2). By TEAE, physical examination (every 6 weeks), laboratory data (every 6 weeks), vital signs (every 6 weeks), ECG (at study close-out visit), pancreatic ultrasound (at Safety was assessed by end-of-study follow-up) and brain MRI (at end-of-study follow-up). Relapses were recorded and EDSS was performed every 6 weeks. Annualized relapse rate (ARR) was analyzed by a Poisson model with the total number of confirmed relapses initiated between the date of randomization and the date of last dose as the response variable, treatment and region as covariates, lo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com