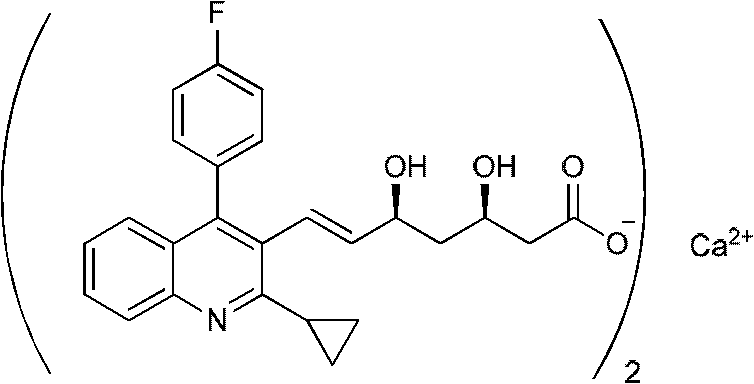
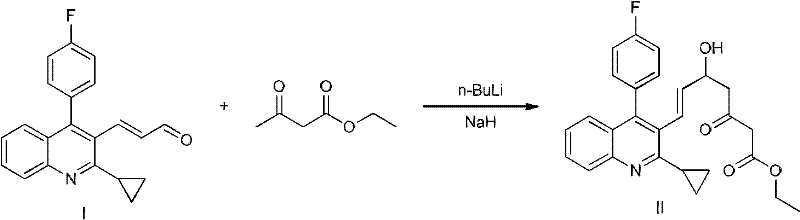
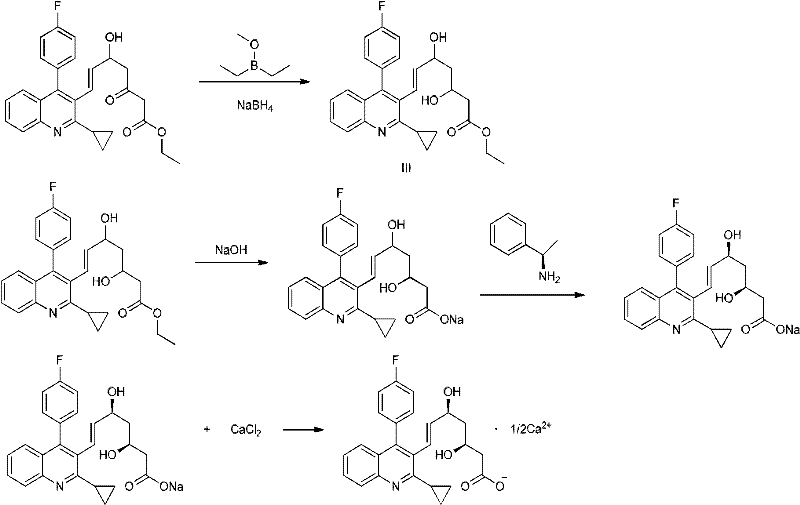
Preparation method of pitavastatin calcium intermediate
A technology of pitavastatin calcium and intermediates, applied in the field of medicine, to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] As shown in the reaction flow formula 1, under the protection of nitrogen, add 300ml of tetrahydrofuran into a 2000ml reaction kettle, add 13.2g (0.33 moles) of 60% sodium hydride, cool to -10°C, and add 39.05% ethyl acetoacetate dropwise from the dropping funnel. g (0.3 moles), after the dropwise addition is complete, continue to stir for 30 minutes, dissolve 40.00 g (0.33 moles) of s-tert-butylsulfinamide in 100 ml of tetrahydrofuran, add it dropwise to the reaction kettle, stir and react for 40 minutes, and the reaction system After cooling to -20°C, 240 ml (0.48 mol) of 2 mol / liter n-butyllithium tetrahydrofuran solution was added dropwise from the dropping funnel, and the stirring reaction was continued for 1 hour. Dissolve (E)-3-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinoline-2-propenal 63.47g (0.2 mol) in 100ml tetrahydrofuran and add it dropwise to the reaction kettle In, continue stirring for 2 hours. 500ml of saturated ammonium chloride solution was added dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com