Preparation method of pitavastatin calcium by recrystallization
A technology of pitavastatin calcium and recrystallization, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
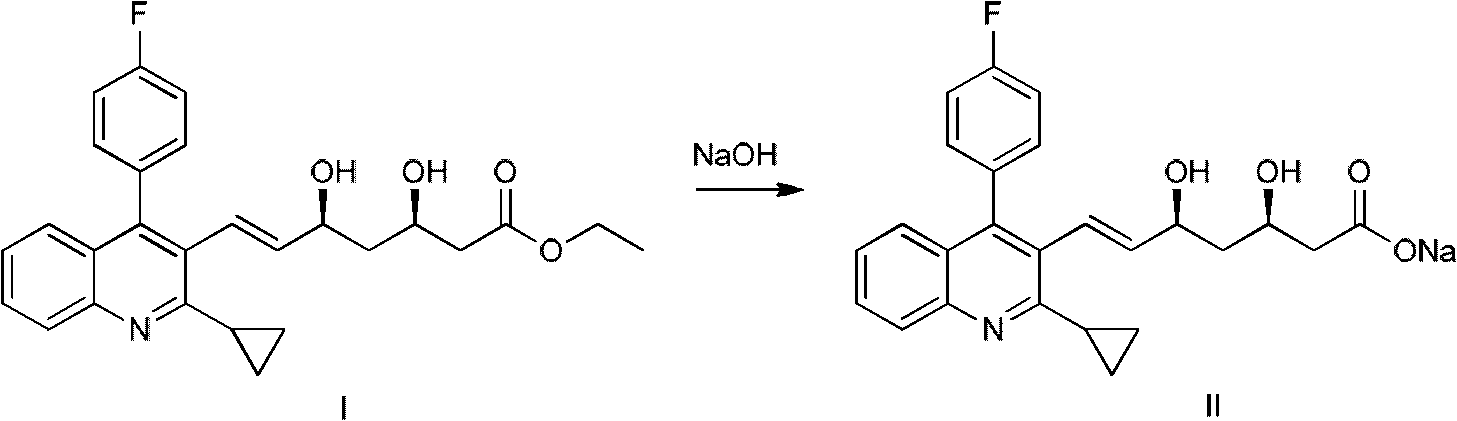
[0027] formula two
[0028] As reaction formula 2, add 134.93g (0.3mol) of compound I in a 2000ml flask, 1500ml absolute ethanol, stir to dissolve, cool to 0-4°C, drop 320ml of 1mol / L sodium hydroxide solution, and continue stirring for 3 hours to complete the hydrolysis reaction. Evaporate the solvent under reduced pressure, add 2000ml of water, stir to dissolve, and prepare pitavastatin sodium salt solution.
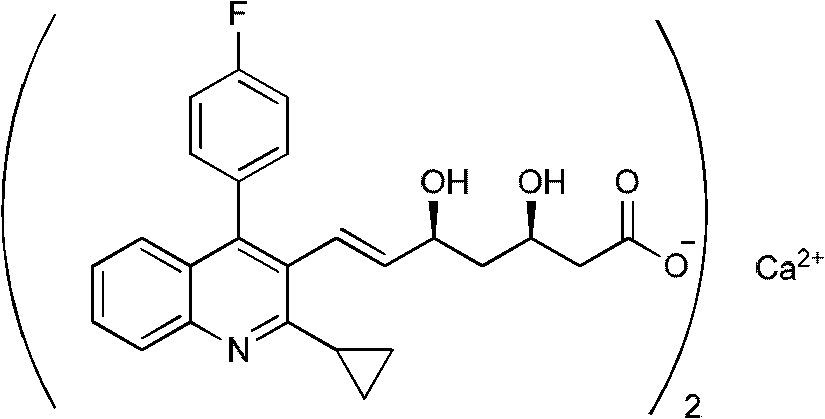
[0029] Add 16.65 g (0.15 mol) of calcium chloride 100 ml aqueous solution dropwise to the prepared pitavastatin sodium salt solution, continue stirring for 12 hours, filter, and dry to obtain pitavastatin calcium 126.8 g, yield 96%, HPLC purity 94% .
Embodiment 2
[0031] Add 134.93g (0.3mol) of compound I in a 2000ml flask, 1500ml absolute ethanol, stir to dissolve, cool to 0-4°C, drop 320ml of 1mol / L sodium hydroxide solution, and continue stirring for 3 hours to complete the hydrolysis reaction . Evaporate the solvent under reduced pressure, add 2000ml of water, stir to dissolve, and prepare pitavastatin sodium salt solution.
[0032] Add 400ml of tetrahydrofuran to the prepared pitavastatin sodium salt solution, drop in 100ml of aqueous solution of 16.65g (0.15mol) of calcium chloride, continue stirring at -0.08MPa for 12 hours to remove tetrahydrofuran by volatilization, filter, and dry to obtain pitavastatin Calcium 124.22g, yield 94%, HPLC purity 98%.
Embodiment 3
[0034] Add 100ml of tetrahydrofuran to 20g of pitavastatin calcium, stir to dissolve, add 400ml of water, continue to stir at -0.08MPa for 12 hours to volatilize and remove tetrahydrofuran, filter and dry to obtain 18.2g of pitavastatin calcium, yield 91%, HPLC purity 98 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com