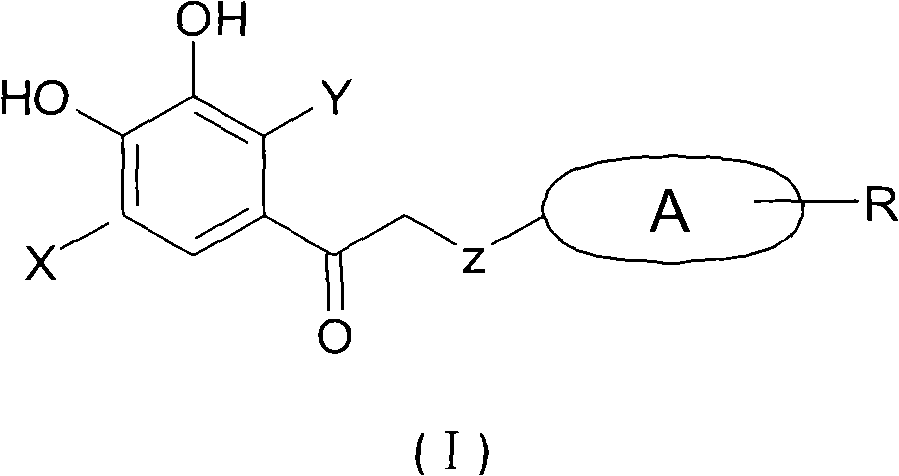
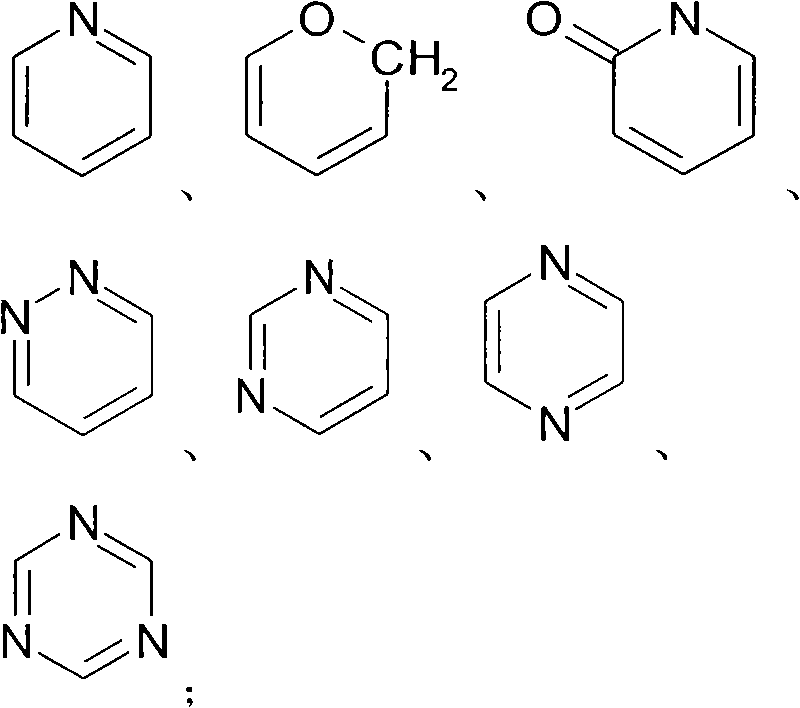
Application of 2-cycloxyl substituted or cyclothio substituted hydroxyacetophenone in treatment of metabolic disease
A technology of metabolism and hydroxyl, which is applied in the field of medicine and can solve problems such as low activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
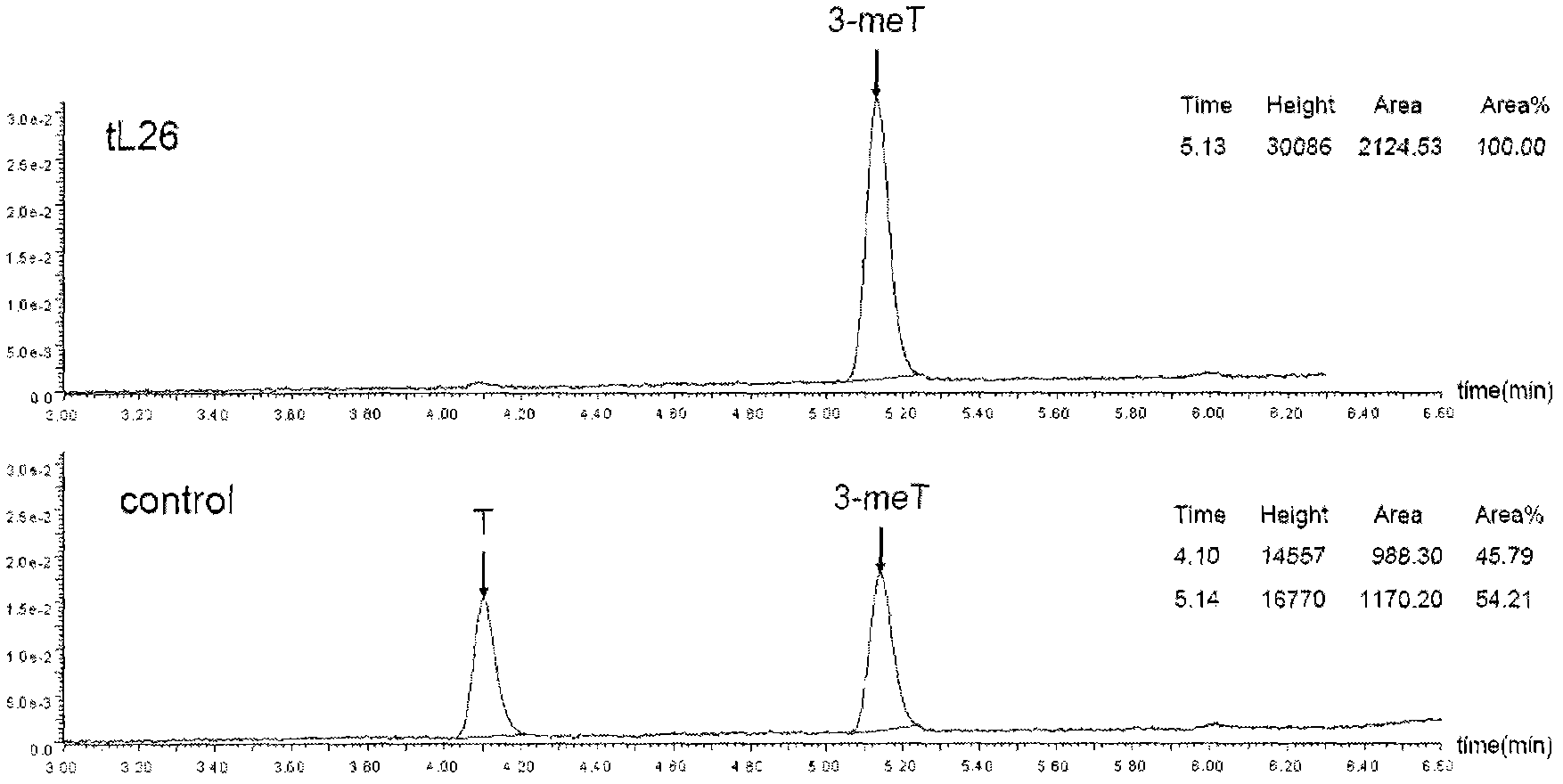
[0154] FTO enzyme activity reaction system:
[0155]
[0156] Add 2 μl substrate 3-meT (1.0 mg ml-1), 2 μl wild-type hFTO (5.0 mg ml-1), 2 μl inhibitor (1.0 mg ml-1) to 100 μl reaction system, and incubate at 16°C for 12 hours. The reaction solution was 50 mM 2-N-morpholinoethanesulfonic acid hydrate (MES solution) pH 6.0, 0.2 mM (NH 4 ) 2 FeSO 4 , 1.0 mM 2-ketoglutarate, 2.0 mM ascorbic acid vitamin C.
[0157] The reaction result detector is a Waters automatic purification LC / MS system detector. The results of the reaction are shown in the accompanying drawings and the table below.
[0158]
[0159]
[0160]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com