Detection method for vestigial protein in amoxicillin prepared by using enzymic method
A technology for the preparation of amoxicillin and enzymatic method, which is applied in the field of pharmaceuticals, can solve the problems of patients' allergies, and achieve the effect of improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] Preparation of the test solution: take 1.0g of each test in Table 2, accurately weigh it, put it in a 25ml volumetric flask, add an appropriate amount of 0.05mol / L phosphate buffer (pH 7.0), dropwise add 0.1mol / L sodium hydroxide solution, shake while adding dropwise, add dropwise until the test product is completely dissolved (solution is clear), and dilute to 25ml with 0.05mol / L phosphate buffer solution (pH7.0), that is, it is obtained.
[0078] Precisely measure 100 μL of each of the above solutions and inject them into a liquid chromatograph, record the peak area, and measure the content of residual protein by the external standard method. The results are shown in Table 2
[0079] Table 2 Determination results of amoxicillin raw materials
[0080]
[0081] *Test results below the limit of quantification are reported as "∠LOQ".
experiment example
[0083] 3. Methodological validation
[0084] 3.1 Exclusivity
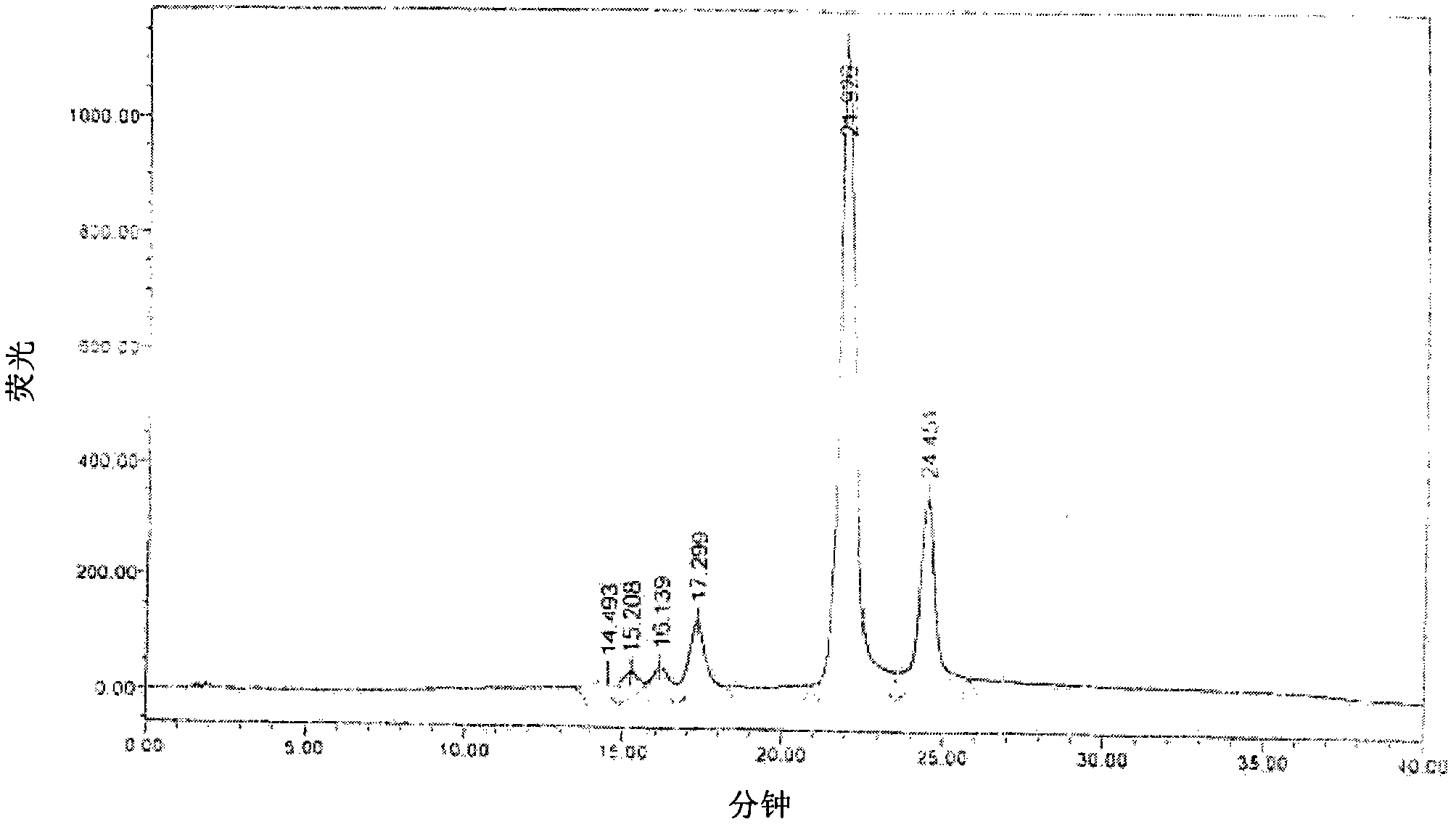
[0085] By measuring the HPLC chart of penicillin G acyltransfer mold solution, blank solution, test solution, test sample recovery rate by the method in the above-mentioned embodiment, refer to Figure 2 to Figure 7 ,Depend on Figure 2 to Figure 7 It can be seen that the main amoxicillin peak does not interfere with the determination of the protein standard peak.
[0086] 3.2 Linear:
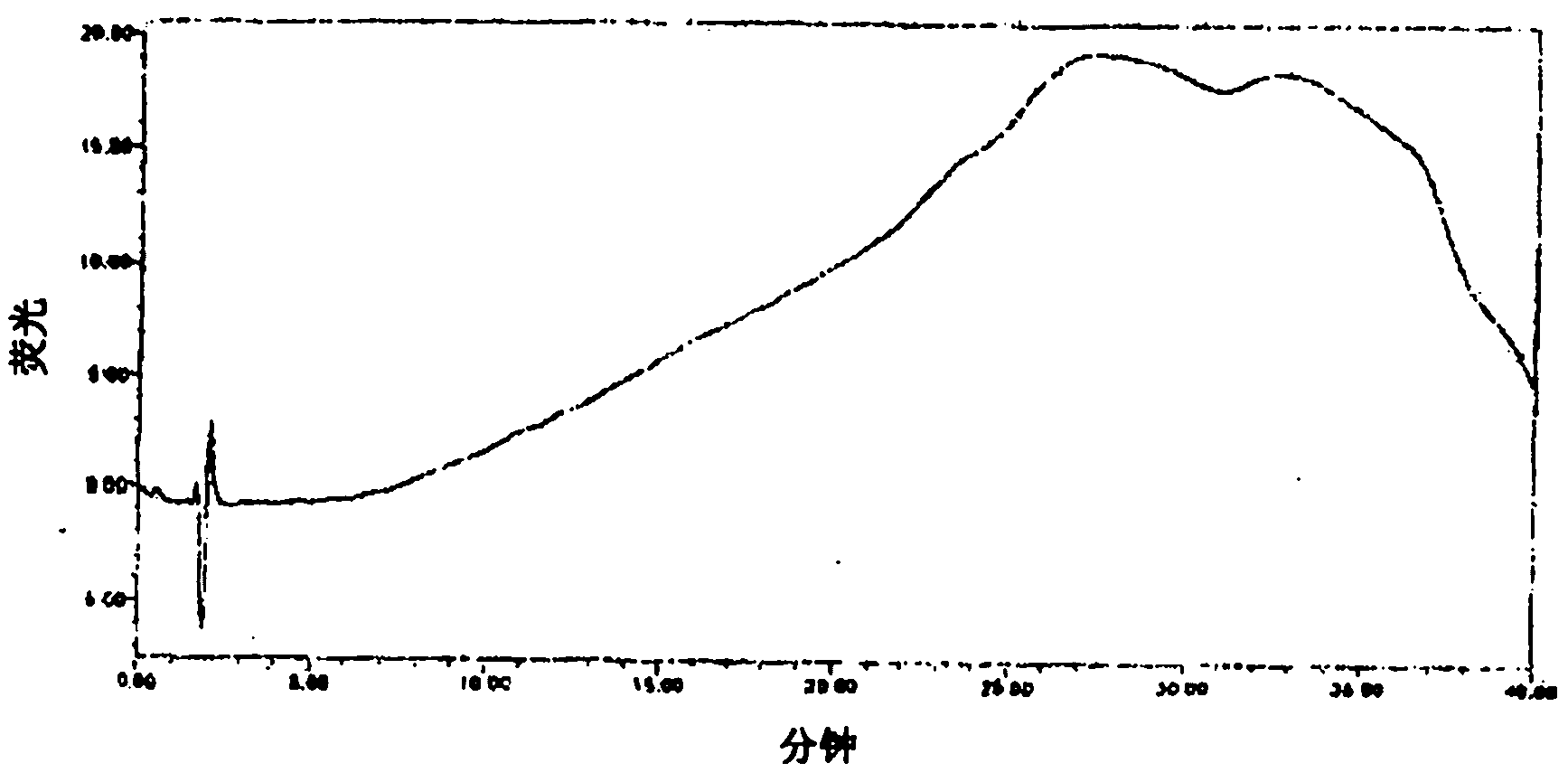
[0087] In the range of protease content from 0.0153 μg / mL to 15.3 μg / mL, the linear relationship is good ( Image 6 ), linear equation: y=18454X-118735, correlation coefficient, 0.9999.
[0088] 3 Detection and quantification limits
[0089] The limit of detection is calculated as S / N=3, LOD=0.072 ppm; the limit of quantification is calculated as S / N=10, LOQ=0.22 ppm.
[0090] 4 Stability of the solution
[0091] 4.1 Stability of standard solutions
[0092] Take about 80mg of protein standard, dissolve and dilute to 100mL wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com