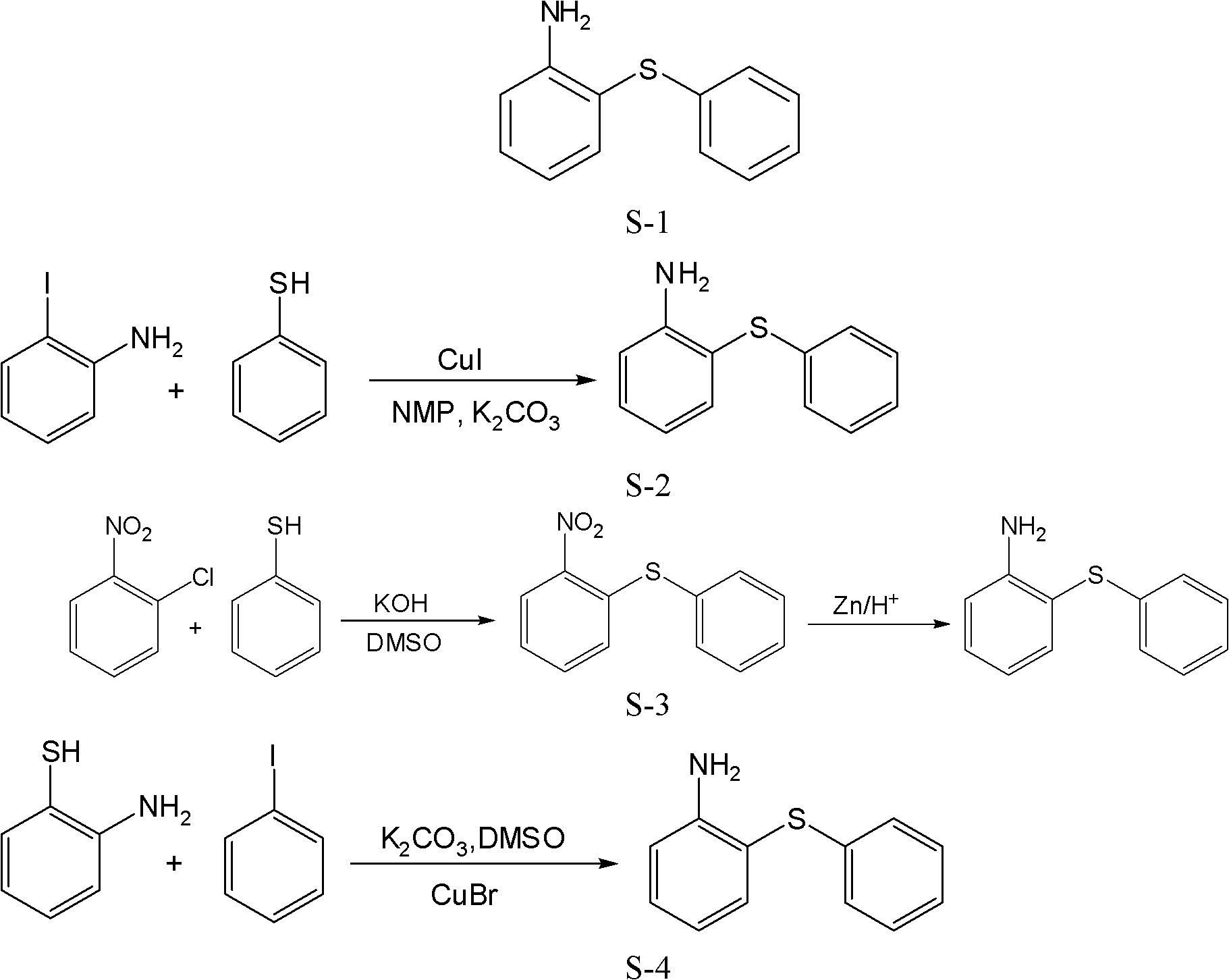
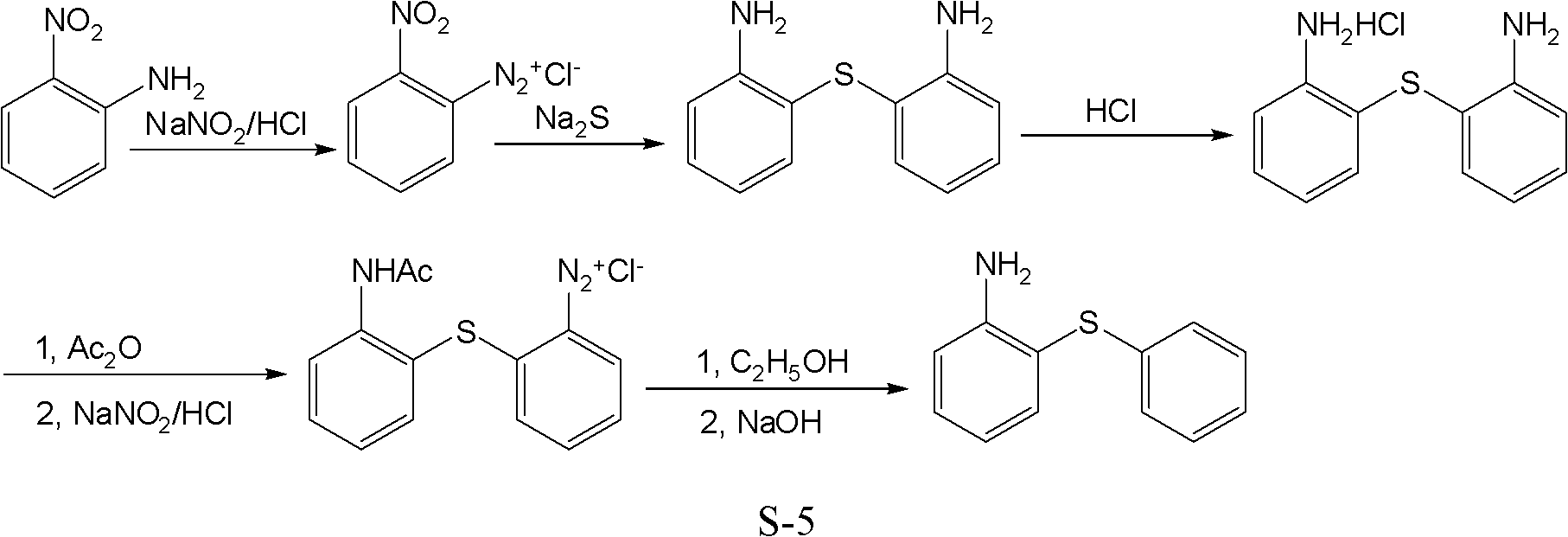
Preparation method of 2-aminophenyl phenyl sulfide
A technology of aminodiphenyl sulfide and diaminodiphenyl sulfide, which is applied in the field of synthesis of 2-aminodiphenyl sulfide, can solve the problems of strong thiophenol odor, environmental pollution, and high price of starting materials, and achieve low cost , easy to obtain raw materials and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, a kind of preparation method of 2-amino diphenyl sulfide, take o-nitroaniline, hydrochloric acid, sodium nitrite, sodium sulfide, acetic anhydride, ethanol, sodium hydroxide etc. as raw materials, carry out the following steps successively:
[0044] Step 1), prepare diazonium salt:
[0045] 13.8g (0.1mol) of o-nitroaniline, 73g of hydrochloric acid solution (11g, 0.3mol) with a mass solubility of 15% were put into a 250ml flask, cooled to 5°C, and 30ml of aqueous sodium nitrite ( 7.9 g, 0.115 mol), adjust the dropping rate to keep the reaction temperature at 5-10 °C, drop after 2 h, continue to stir at 5 °C for 30 min after the dropping is completed, and stop the reaction.
[0046] Step 2), prepare 2,2'-diaminodiphenyl sulfide:
[0047] 150ml of Na with a mass concentration of 17.5% 2S solution (0.4mol) and 12g (0.3mol) NaOH were put into a 500ml flask, heated to 45°C, and all the diazonium salt solutions obtained in step 1) were added dropwise with stir...
Embodiment 2~10
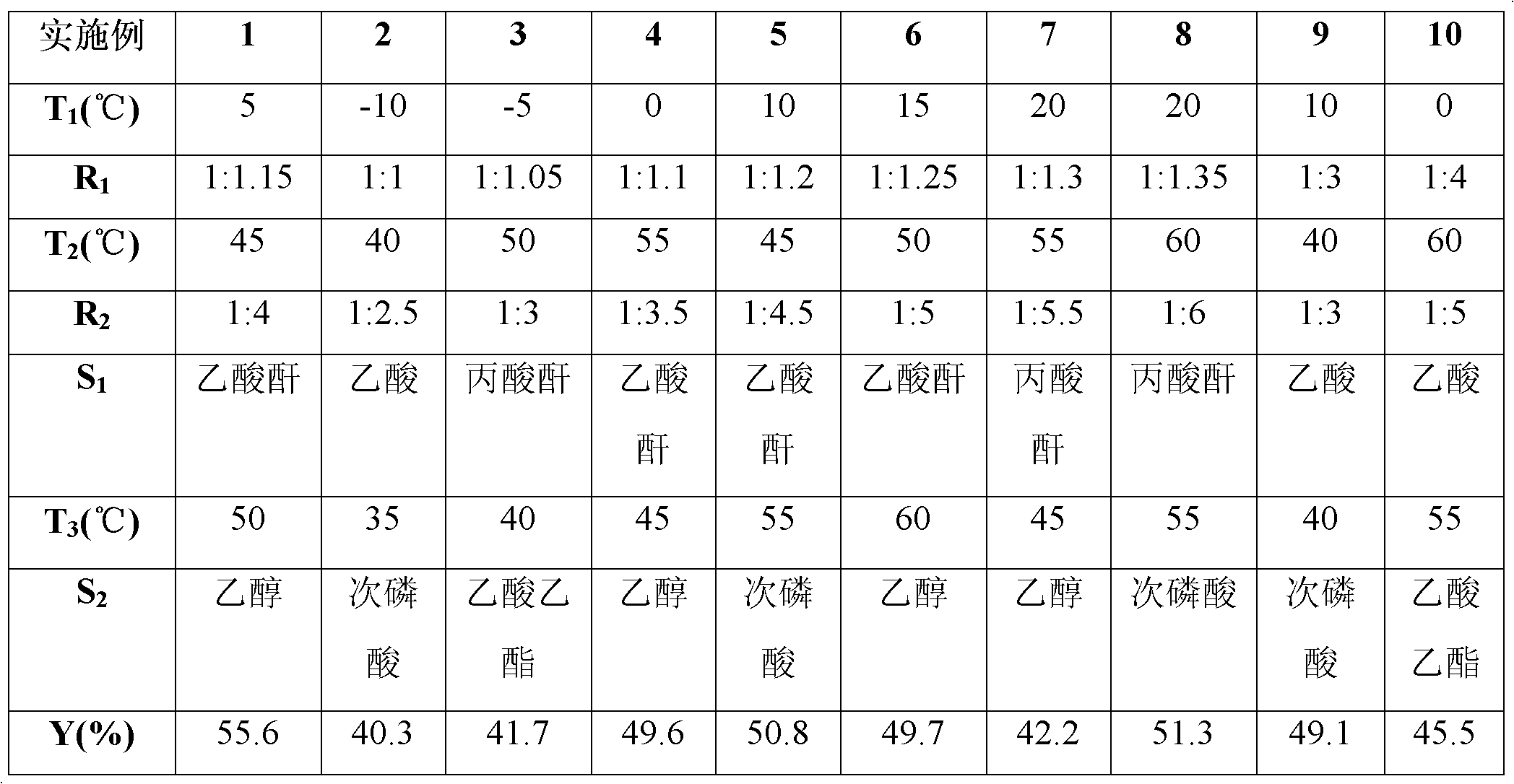
[0053] Change the following reaction conditions in steps 1) to 4) of Example 1:
[0054] In step 1), the diazotization reaction temperature (abbreviated as T 1 ), the mol ratio of o-nitroaniline and sodium nitrite in step 1) (referred to as R 1 ), step 2) in the thioetherification reaction temperature (referred to as T 2 ), the mol ratio of o-nitroaniline and sodium sulfide in step 2) (referred to as R 2 ), step 3) in acylation reaction reagent (referred to as S 1 ), step 4) in the denitrification reaction temperature (referred to as T 3 ), step 4) in the denitrification reaction reagent (referred to as S 2 ) to obtain Examples 2 to 10, and the molar ratios between the remaining reaction raw materials are the same as those of Example 1; thereby obtaining the yield of the corresponding 2-aminodiphenyl sulfide (referred to as Y). The specific content and data results are shown in Table 1.
[0055] Table 1
[0056]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com