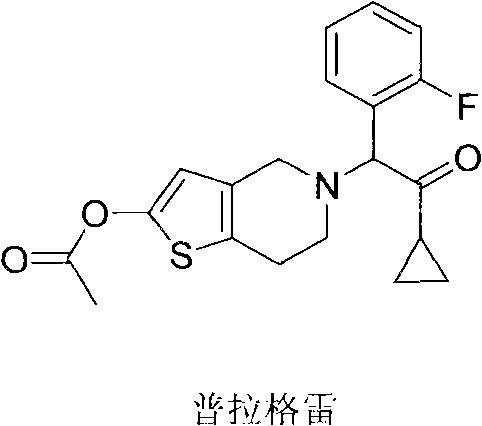
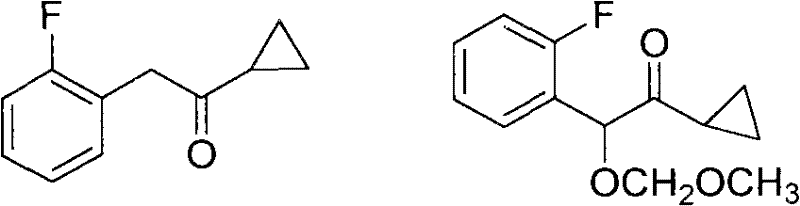
Method for preparing prasugrel intermediate with one-pot method
An intermediate, fluorophenyl technology, which is applied in the field of one-pot preparation of prasugrel intermediates, can solve the problems of increased difficulty in the preparation of prasugrel, difficult storage, difficult preparation, etc., and achieves low cost, simplified reaction, and post-production The effect of simple processing technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 The method for preparing the prasugrel intermediate in one pot method comprises the following steps:
[0031] (1) Synthesis of 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy)-N-methylacetamide:
[0032] In a 50mL three-necked flask, with toluene as solvent, under the protection of nitrogen, 2-(2-fluorophenyl)-2-(methoxymethoxy)acetic acid (3mmol, 0.65g) and PCl 3 (1.5mmol, 0.21g), NH(CH 3 OCH 3 ) (15mmol, 0.9g) was reacted at 0°C for 8h, detected by TLC (thin layer chromatography), and 2-(2-fluorophenyl)-N-methoxy-2-(methoxy Methoxy)-N-methylacetamide.
[0033] 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy)-N-methylacetamide plus saturated NaHCO 3 The aqueous solution was quenched, the organic phase was extracted with ether, dried with anhydrous magnesium sulfate, and finally separated by column chromatography to obtain the pure product 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethyl Oxy)-N-methylacetamide 0.7 g, yield: 91%.
[0034] 1 HNMR (CDCl 3 ,...
Embodiment 2
[0046] Embodiment 2 The method for preparing the prasugrel intermediate in one pot method comprises the following steps:
[0047] (1) Synthesis of 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy)-N-methylacetamide:
[0048] In a 50mL three-necked flask, using tetrahydrofuran as a solvent, under nitrogen protection, 2-(2-fluorophenyl)-2-(methoxymethoxy)acetic acid (3mmol, 0.65g) and PCl 3 (1.5mmol, 0.21g), NH(CH 3 OCH 3 ) (18mmol, 1.1g) was reacted at 25°C for 8.5h, and detected by TLC, after the reaction of the raw materials, 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy) was obtained -N-methylacetamide.
[0049] 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy)-N-methylacetamide plus saturated NaHCO 3 The aqueous solution was quenched, the organic phase was extracted with ether, dried with anhydrous magnesium sulfate, and finally separated by column chromatography to obtain the pure product 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethyl Oxy)-N-methylacetamide 0.75 g, yield:...
Embodiment 3
[0054] Embodiment 3 One-pot method for preparing the method of prasugrel intermediate, comprises the following steps:
[0055] (1) Synthesis of 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy)-N-methylacetamide:
[0056] In a 50mL three-necked flask, with dichloromethane as solvent, under the protection of nitrogen, 2-(2-fluorophenyl)-2-(methoxymethoxy)acetic acid (3mmol, 0.65g) and PCl 3 (3mmol, 0.41g), NH(CH 3 OCH 3 ) (9mmol, 0.55g) was reacted at 10°C for 9h, and detected by TLC. After the reaction of the raw materials, 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy)- N-methylacetamide.
[0057] 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethoxy)-N-methylacetamide plus saturated NaHCO 3 The aqueous solution was quenched, the organic phase was extracted with ether, dried with anhydrous magnesium sulfate, and finally separated by column chromatography to obtain the pure product 2-(2-fluorophenyl)-N-methoxy-2-(methoxymethyl Oxy)-N-methylacetamide 0.75 g, yield: 97%.
[0058] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com