Adsorbent for removing fluorine ions in water body and preparation method and application method of adsorbent
An application method and adsorbent technology, applied in the direction of chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the problems of secondary pollution adsorption performance, narrow pH application range, complex preparation process, etc., and achieve physical Strong chemical stability, low equipment requirements and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1 Preparation of iron-aluminum composite oxide nanoparticle adsorbent
[0019] Take 0.5g of sodium lauryl sulfate and add it to 100mL of deionized water, place it in a 500mL four-neck flask, and add 1mol / L NaOH (about 1-2 drops) dropwise to make the pH value about 11. Take 3.89g of FeCl 3 ·6H 2 O and 2.29 g FeCl 2 4H 2 O (the molar ratio is 1.25:1) is miscible in 50mL of 0.25mol / L hydrochloric acid solution, and then added dropwise to the four-necked flask reactor, and N 2 , fully stirred at 80°C (240-260r / min) for 1.5h, and adjusted the pH with NaOH to maintain the pH at about 11.0. After cooling, standing, and washing with deionized water for 3 times, ultrasonic for 10 minutes, then add 5.73gAl 2 (SO 4 ) 3 18H 2 O, adjust the pH to 5.0, blow nitrogen, stir at 70°C (240-260r / min) for 1.5 hours, cool, let stand, and wash with deionized water for 3 to 6 times, and put the washed product into an oven at 60°C Dry to constant weight and dry to obtain the...
Embodiment 2
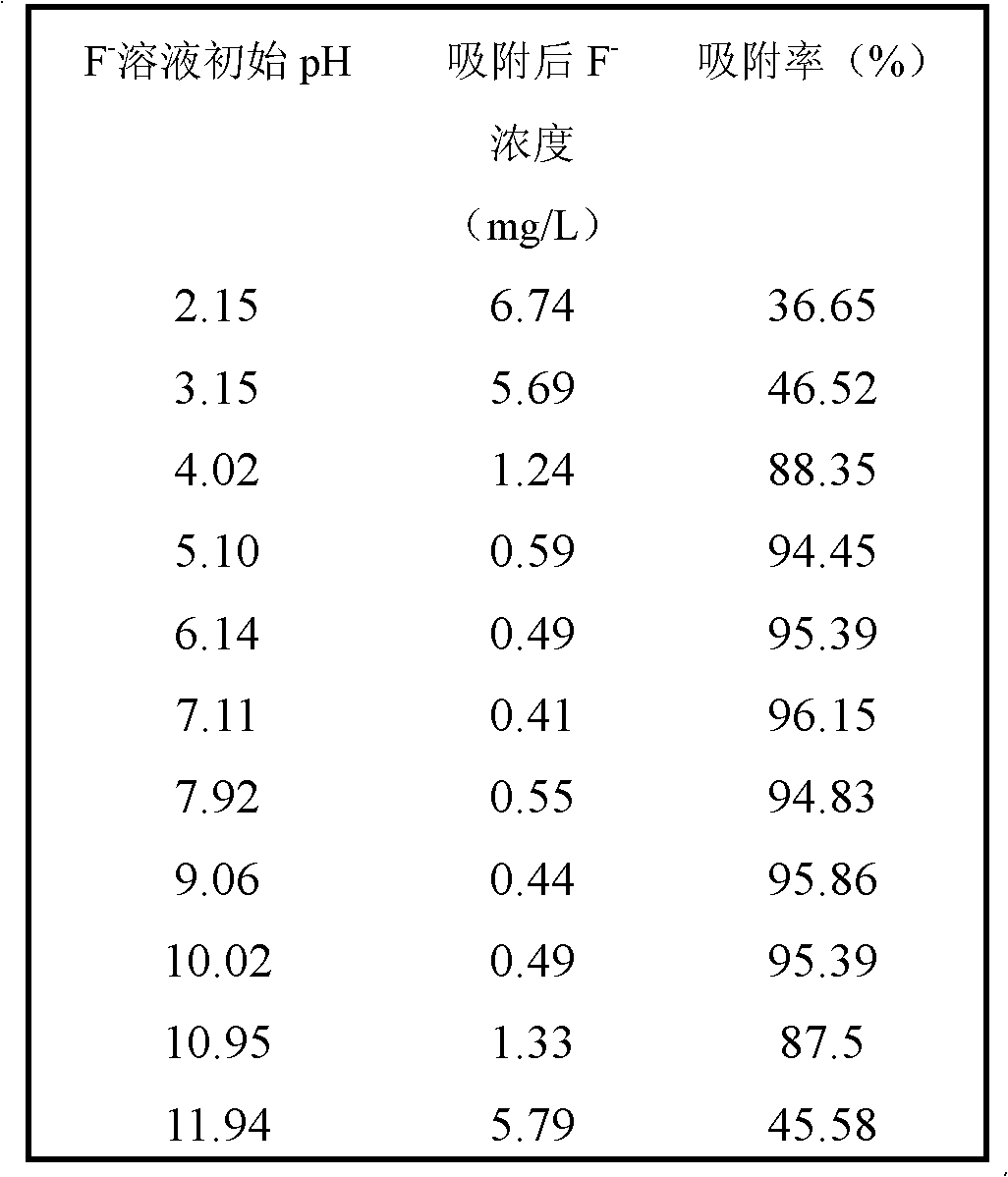
[0022] At room temperature, use 100 mg of the adsorbent prepared in Example 1 for 100 mL of 10.64 mg L -1 The fluorine-containing solution was subjected to adsorption reaction, and the reaction time was 7 hours; the initial pH of the fluoride-containing solution was adjusted to 2.15, 3.15, 4.02, 5.10, 6.14, 7.11, 7.92, 9.06, 10.02, 10.95, and 11.94. After adsorption, the solution was filtered with sand core, and the concentration of fluoride ion in the filtrate was determined by fluoride ion selective electrode method. Table 1 shows the fluoride ion concentration and adsorption rate after adsorption at different initial pH values of the solution. It was found that in the range of pH 4-10, the removal rate of fluorine ion by the adsorbent reaches about 90%.
[0023] Table 1
[0024]
Embodiment 3
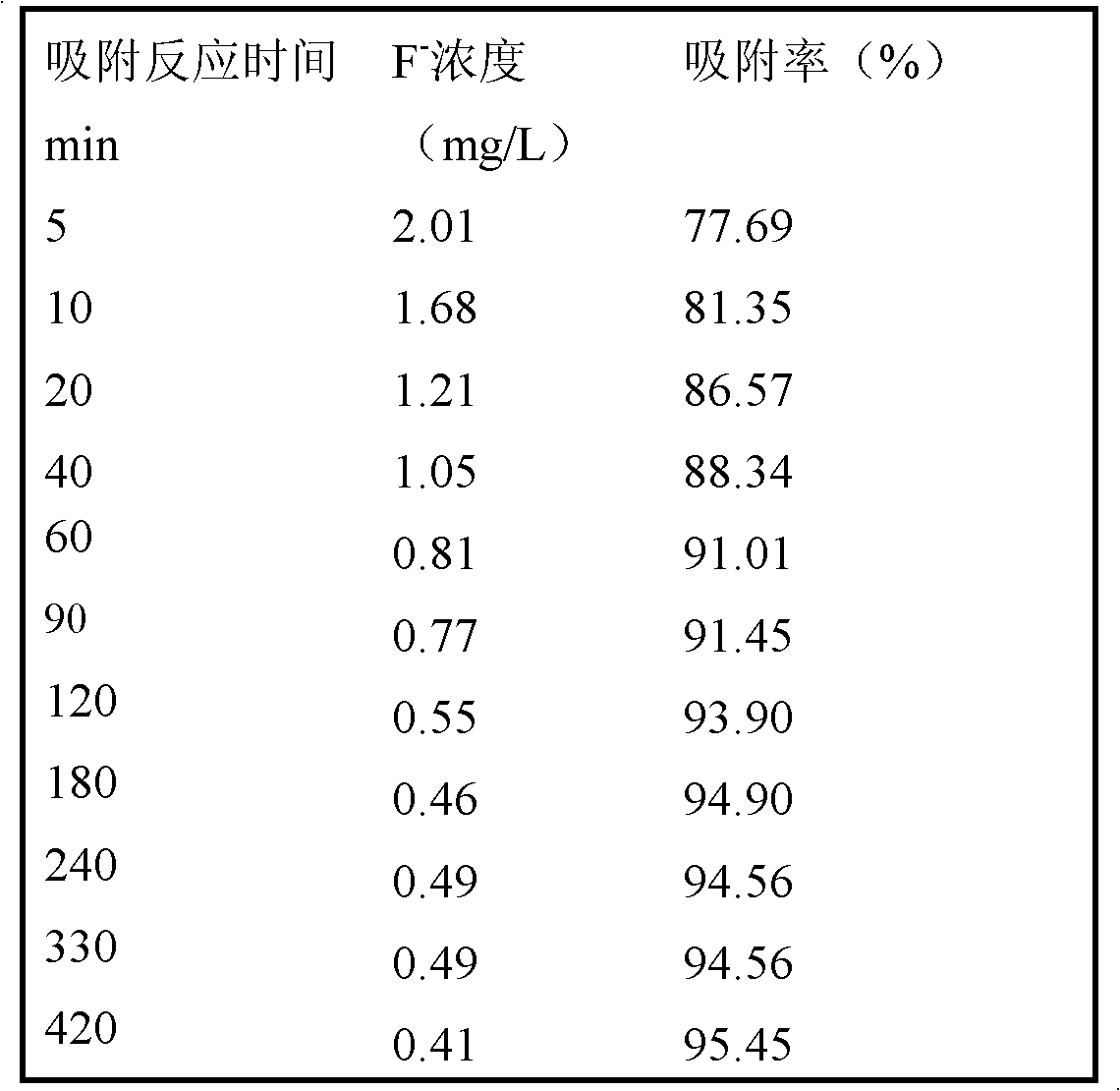
[0026] At room temperature, with 100 mg of the adsorbent prepared in Example 1, the initial pH of the 100 mL solution is 7.0, and the F-concentration is 9.01 mg L -1 The solution undergoes adsorption reaction, wherein the reaction time is controlled at 5, 10, 20, 40, 60, 90, 120, 180, 240, 330, 420 min. After the reaction, the mixed solution was filtered and collected in a dry beaker, and the concentration of fluoride ions in the filtrate was determined by a fluoride ion selective electrode method. Table 2 shows the solution concentration and adsorption rate of fluoride ions under different reaction times.
[0027] It can be seen that the adsorbent of the present invention has a fast adsorption rate for fluorine ions, and the adsorption equilibrium is reached after 2 hours of reaction, and the adsorption rate has reached about 94%. (GB5749-2006).
[0028] Table 2
[0029]
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com