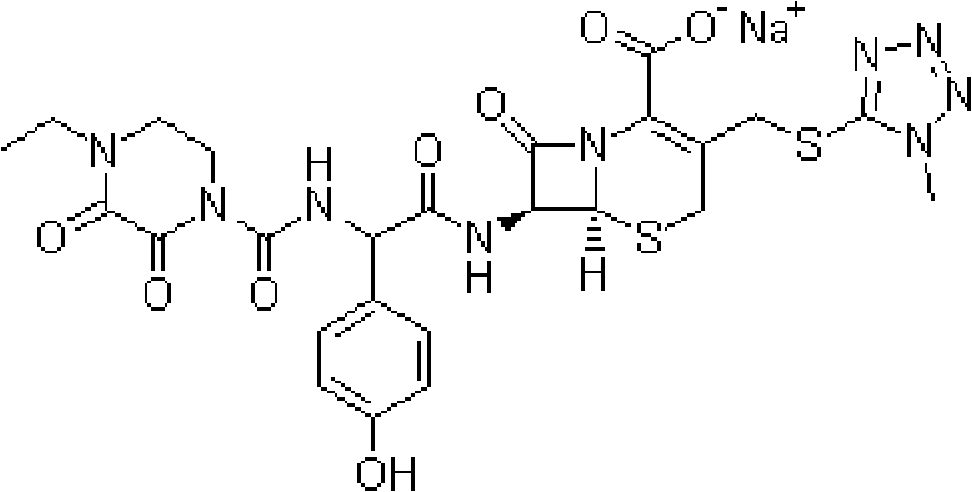
Injection powder and injection preparation of cefoperazone sodium-tazobactam combination
A kind of injection powder, tazobactam sodium technology, applied in the field of cefoperazone sodium and tazobactam sodium composition injection powder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Cefoperazone Sodium and Tazobactam Sodium Composition Powder for Injection provided by the present invention
[0024] Accurately weigh 800g of cefoperazone sodium, 200g of tazobactam sodium, and 10g of lidocaine hydrochloride, pass through a 100-mesh sieve, mix, fine filter, fill, seal, and then prefreeze at -55°C for 1 hour The sublimation temperature is -35°C, the sublimation time is 5h; the drying temperature is 30°C, and the drying time is 11h, and freeze-drying is carried out to obtain the cefoperazone sodium and tazobactam sodium composition powder for injection.
Embodiment 2
[0025] Example 2 Cefoperazone Sodium and Tazobactam Sodium Composition Powder for Injection provided by the present invention
[0026] Accurately weigh 1600g of cefoperazone sodium, 400g of tazobactam sodium, and 100g of lidocaine hydrochloride, pass through an 80-mesh sieve, after mixing, fine filter, fill, seal, and then prefreeze at -30°C for 8 hours The sublimation temperature is -10°C, the sublimation time is 20h; the drying temperature is 15°C, and the drying time is 15h, and freeze-drying is carried out to obtain the cefoperazone sodium and tazobactam sodium composition powder for injection.
Embodiment 3
[0027] Example 3 Cefoperazone Sodium and Tazobactam Sodium Composition Powder for Injection provided by the present invention
[0028] Accurately weigh 800g of cefoperazone sodium, 200g of tazobactam sodium, and 50g of lidocaine hydrochloride, pass through a 90-mesh sieve, mix, fine filter, fill, seal, and then prefreeze at -45°C for 3 hours The sublimation temperature is -27°C, the sublimation time is 10h; the drying temperature is 42°C, and the drying time is 7h, and freeze-drying is carried out to obtain the cefoperazone sodium and tazobactam sodium composition powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com