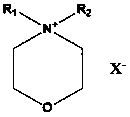
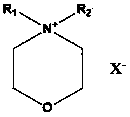
Application of morpholine-containing ionic compound serving as catalyst for synthesizing cyclic carbonate
A cyclic carbonate and morpholine technology is applied in the field of catalysts for synthesizing cyclic carbonates, and achieves the effects of low energy consumption, environmental friendliness and clean process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In 50 milliliters of stainless steel autoclaves, add 9.25g (0.1mol) epichlorohydrin, 0.32g (1%mol) N-methyl-N-dodecylmorpholine ammonium bromide (its preparation is with reference to N- For the preparation of methyl-N-hexadecylmorpholine ammonium bromide, only dodecyl bromide is used instead of hexadecane bromide), the reaction kettle is sealed, and carbon dioxide with a pressure of 0.1MPa is filled, and the temperature is raised to 90°C. Reacted for 24 hours, cooled to room temperature, and slowly released the pressure. The resulting liquid was distilled under reduced pressure to obtain the product 4-chloromethyl-[1,3]dioxolane-2-one—a cyclic carbonate, which was GC-MS and NMR detection, the reaction selectivity is 100%, and the yield is 99.9%. The NMR data are as follows: 1 HNMR (CDCl 3 , 300MHz): δ (ppm): 3.78 (dd, J =3.6,12.3Hz,1H); 3.86 (dd, J =4.9, 12.3Hz ,1H); 4.44(dd, J =5.7,8.8Hz,1H); 4.64(t, J =8.7Hz,1H); 5.02~5.08 (m,1H)
Embodiment 2
[0029] Same as the reaction conditions and detection method in Example 1, only the catalyst used is changed to 0.04g (1‰ mol) N-methyl-N-decylmorpholine ammonium iodide (the preparation refers to N-decyl-N - Preparation of methyl-morpholine ammonium chloride by replacing the chlorine of N-decyl-N-methyl-morpholine ammonium chloride with iodine) to obtain 4-chloromethyl-[1,3]dioxane Pentan-2-one, the reaction selectivity is 100%, and the yield is 98.6%.
Embodiment 3
[0031] Same as the reaction conditions and detection method in Example 1, only the catalyst used is changed to 0.06g (2‰ mol) N-methyl-N-tetradecylmorpholine ammonium chloride (its preparation refers to N-methyl- Preparation of N-hexadecylmorpholine ammonium bromide, only tetradecyl chloride was replaced by hexadecane bromide) to obtain 4-chloromethyl-[1,3]dioxolan-2-one, The reaction selectivity was 100%, and the yield was 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com