Novel method for preparing ezetimibe key intermediate
A technology of ezetimibe and a new method, applied in the production of bulk chemicals, organic chemistry, etc., can solve problems such as increased costs and long routes, and achieve the effects of cost saving, simple processing, and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] The present invention will be described in further detail below through the examples, and the purpose of the examples is to illustrate rather than limit.
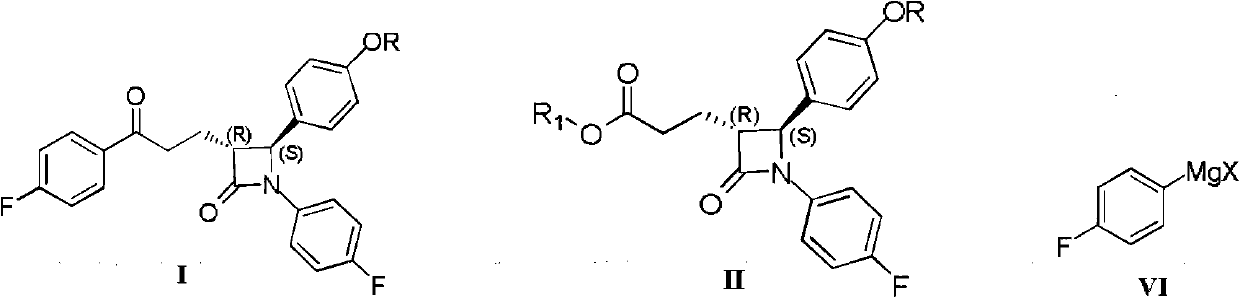
[0043] The present invention relates to the method for preparing compound I as shown in Scheme 1:
[0044] plan 1
[0045]
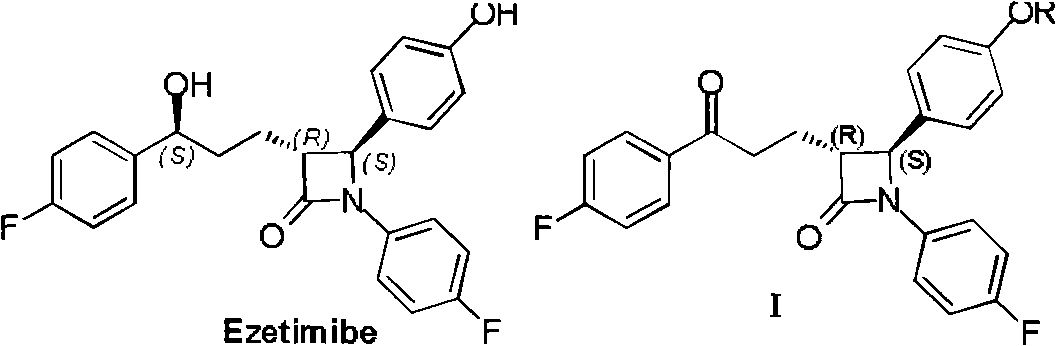
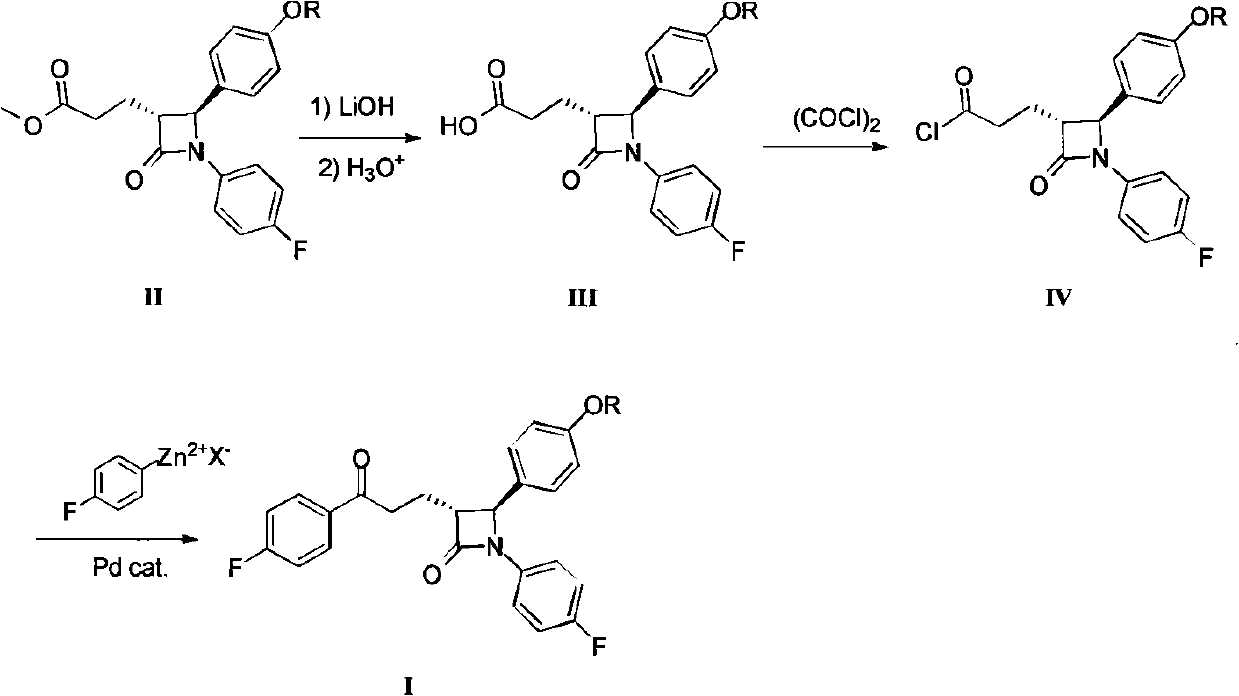
[0046] Specifically, compound II reacts with 4-fluorophenyl Grignard reagent in toluene under the action of triethylamine to obtain compound I. Compound II is a known compound and can be prepared by known methods.
[0047] (3R,4S)-1-(4-fluorophenyl)-3-[3-(4-fluorophenyl)-3-oxopropyl]-4-benzyloxyphenyl-2-azetidine Preparation of ketone (I)
[0048] method one:
[0049] Add 10 mL of dry toluene to a dry three-necked flask under nitrogen protection, cool to -30 degrees, slowly add 4-fluorophenyl Grignard reagent (2M ether solution, 2.08mL, 4.16mmol, 2eq), and then slowly add triethyl Amine (1.73mL, 12.5mmol, 6eq), dropwise completed, stirred for 15 minutes.
[0050] 5 mL of toluene solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com