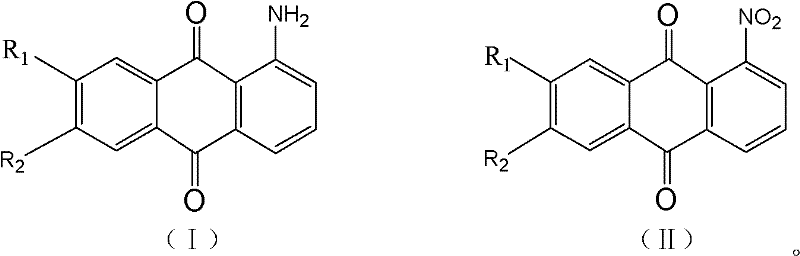
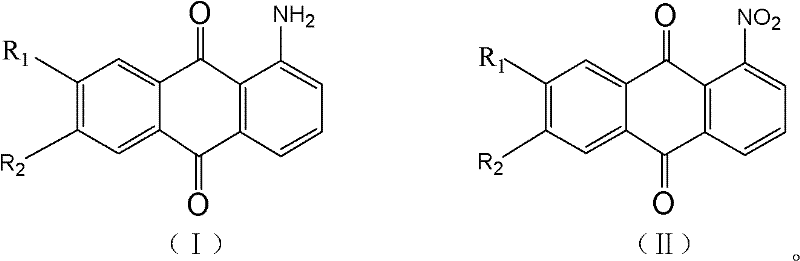
Method for preparing high-purity 1-aminoanthraquinone through catalytic hydrogenation
A technology of aminoanthraquinone and nitroanthraquinone, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of no industrial value and high cost, and achieve good industrial value and solvent use. The effect of less quantity, low equipment requirements and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Insert electromagnetic stirring into a 2L normal-pressure hydrogenation glass reactor, and add 100 g of 1-nitroanthraquinone with a purity of 99.0%, 600 g of N, N-dimethylformamide (DMF), 3% palladium / Carbon catalyst 5.0g, feed hydrogen into the system, carry out hydrogenation reaction at room temperature. After 3 hours of hydrogenation (according to theoretical calculation, the amount of hydrogen intake is 1.18 mol), the flow of hydrogen was stopped. After the reducing solution was filtered to remove the catalyst, it was stirred in the air for 1 h, and placed in a freezer for 4 h to crystallize. Then the crystals precipitated from the reducing solution were filtered, weighed 63.5 g after drying, and its purity was detected by liquid chromatography. The content of 1-aminoanthraquinone was 99.4%. Apply the mother liquor and the catalyst mechanically, add 80 g of 1-nitroanthraquinone, repeat the above reaction and post-treatment process, and carry out liquid chromatogra...
Embodiment 2
[0020] In a 2L electromagnetically stirred hydrogenation pressure reactor, add 240g of 1-nitroanthraquinone with a purity of 99.0%, 600g of N,N-dimethylformamide (DMF), and 18.0g of Raney Ni catalyst, and pass through the system Inject hydrogen, and carry out hydrogenation reaction at 65°C. After 4 hours of hydrogenation, hydrogen flow was stopped. After the reducing solution was filtered to remove the catalyst, it was stirred in the air for 1 h and then stood at room temperature for 8 h to crystallize. Then the crystals precipitated from the reducing solution were filtered, dried and weighed to be 158g. The purity was detected by liquid chromatography, and the content of 1-aminoanthraquinone was 99.4%. Apply the mother liquor and catalyst mechanically, add 180g of 1-nitroanthraquinone, repeat the above reaction and post-treatment process (wherein, 3.0g of catalyst is added when applying mechanically for the seventh time), and carry out liquid phase analysis for each precipit...
Embodiment 3
[0022] In a 2L electromagnetically stirred hydrogenation pressure reactor, add 240g of 1-nitroanthraquinone with a purity of 99.0%, 500g of N,N-dimethylformamide (DMF), 300g of xylene, and 18.0g of Raney Ni catalyst, Introduce hydrogen into the system to carry out hydrogenation reaction at 80°C. After 3 hours of hydrogenation, hydrogen flow was stopped. After the reducing solution was filtered to remove the catalyst, it was stirred in the air for 1 h and then stood at room temperature for 6 h to crystallize. Then the crystals precipitated from the reducing solution were filtered, weighed 179g after drying, and its purity was detected by liquid chromatography. The content of 1-aminoanthraquinone was 99.5%. Apply the mother liquor and catalyst mechanically, add 204g of 1-nitroanthraquinone, repeat the above reaction and post-treatment process, and perform liquid chromatography detection on each precipitated crystal obtained by applying mechanically 8 times, and the content of 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com