Method for catalytic synthesis of tributyl citrate by utilizing immobilized p-toluenesulfonic acid
A technology of tributyl citrate and p-toluenesulfonic acid is applied in the chemical industry, and can solve the problems of complex post-treatment process, many catalyst side reactions, complex preparation process and the like, achieve easy recovery and reuse, and improve the efficiency of esterification reaction. , The effect of simplifying the post-processing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1) Preparation of immobilized p-toluenesulfonic acid
[0023] Take 2.5g of sodium alginate, 5g of polyvinyl alcohol and 1.5g of p-toluenesulfonic acid, add it to 100mL of deionized water, mix well, heat it in a constant temperature water bath at 80°C to completely dissolve it, and cool it to about 45°C with a constant temperature The flow pump drops it into 100 mL of calcium chloride solution with a mass percentage of 1.5% at a speed of 60 rpm and continuously stirs it slowly. Then take out and filter to obtain immobilized p-toluenesulfonic acid.
[0024] 2) Esterification reaction
[0025] Add 308g n-butanol, 200g citric acid, immobilized p-toluenesulfonic acid prepared in step 1) into a 500mL three-necked flask equipped with electromagnetic stirring, thermometer, reflux condenser, and water separator, heat under reflux at 130°C and stir, The water produced by the reaction is separated from the water separator, and the reaction is stopped when it is no water and then...
Embodiment 2-4
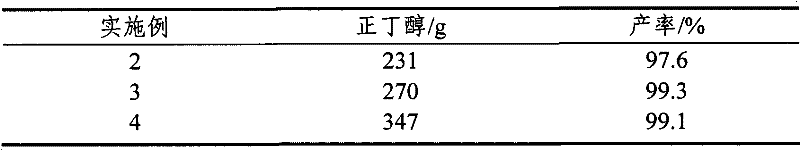
[0029] Only change the amount of n-butanol, and the rest are the same as in Example 1. The results are shown in Table 1.
[0030] Table 1
[0031]
Embodiment 5-7
[0033] Only the esterification temperature was changed, and the rest were the same as in Example 1. The results are shown in Table 2.
[0034] Table 2
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com