Application of ALC1 to preparation of leukaemia drug resistance reversing agent and as drug-resistant leukaemia diagnosing reagent
A technology for leukemia and drug resistance, which is applied in pharmaceutical formulations, biochemical equipment and methods, antineoplastic drugs, etc., can solve problems such as inability to effectively predict chemotherapeutic agent resistance, achieve remarkable effects, broad clinical application prospects, and improve The effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Detection of ALC1 in peripheral blood and bone marrow samples
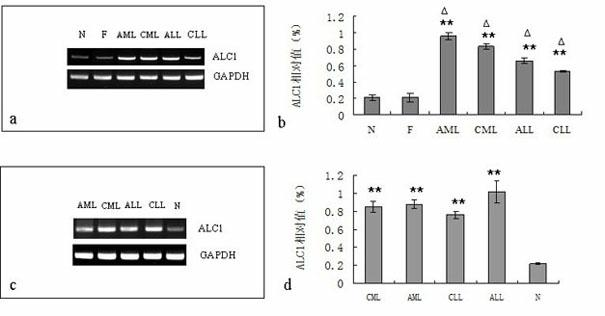
[0026] Peripheral blood and bone marrow were collected from normal people and patients with various types of acute and chronic leukemia (the bone marrow of normal people was obtained from bone marrow donors who matched). In order to compare the expression of ALC1 in the normal and abnormal proliferation of white blood cells, the experiment also selected infection after general surgery. Peripheral blood (total white blood cells > 10×109 / L) was used as a control. Total RNA was extracted, total RNA was quantified, and cDNA was synthesized by reverse transcription according to the kit instructions. The cDNA product was stored at -20°C or used immediately. PCR primers were synthesized by Shanghai Sangong Company: ALC1 (800bp) Sence: 5′-CCTCCTTCTATGATCATGTTG-3′, Antisence: 5′-TGTTCTCATCCCAGGCCTTG-3′; GAPDH (310bp) Sence: 5′-GCCTCAAGATCAGCAAT-3′, Antisence: 5 '-AGGTCCACCACTG ACACGTT-3'. The PCR reaction...
Embodiment 2
[0028] Example 2 Semi-quantitative analysis of ALC1 expression in acute and chronic leukemia drug-resistant cells and non-drug-resistant parental cells
[0029] Human chronic myeloid leukemia acute erythroleukemia cell line K562 and its drug-resistant cell line K562 / ADR (doxorubicin-resistant cell line, 1.0μg / ml doxorubicin culture system maintains drug resistance), human acute myeloid leukemia cells Strain HL-60 and multidrug-resistant cell line HL-60 / ADR (doxorubicin-resistant cell line, 0.5 μg / ml doxorubicin culture system to maintain drug resistance) in RPMI1640 culture medium containing 15% fetal bovine serum at 37°C with 5% CO 2 Cultured in an incubator with saturated humidity, subcultured once every 2-3 days, and cultured for 4 generations before the experiment, all experiments were carried out with cells in the exponential growth phase. Extract total RNA from logarithmic growth phase cells (K562 and K562 / ADR cells, HL-60 and HL-60 / ADR cells) with RNA extraction kit (R...
Embodiment 3
[0031] Example 3 Reversal effect of ALC1 siRNA interference on drug resistance of leukemia cells
[0032] K562 cells and K562 / ADR cells, HL-60 cells and HL-60 / ADR cells were selected for pair verification.
[0033] (1) The half maximal inhibitory concentration (IC) of doxorubicin (ADR) was detected by MTT assay 50 ): K562 cells and K562 / ADR cells in the logarithmic growth phase (1×10 5 / ml), HL-60 cells and HL-60 / ADR cells (2×10 5 / ml) were inoculated in a 96-well culture plate, and each strain of cells was divided into 4 groups: control group (blank group without drug addition), drug group, ALC1 siRNA interference group, (siRNA+drug) group. For siRNA interference, the adenoviral vector (ad-ALC1 siRNA) carrying the ALC1 siRNA fragment that has been successfully constructed is preferred. The cells in each group were added with Ad-mock (MOI 100, blank group, ADR group) and ad-mock diluted with serum-free RPMI-1640. -ALC1 siRNA (MOI 100, siRNA interference group, siRNA+drug gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com