Medicament composition for treating diabetes and application of medicament composition
A composition and diabetes technology, applied in the field of medicine, can solve problems such as subcutaneous bleeding, aggravated ischemia, and repeated injections, and achieve the effects of improving blood lipid levels, good therapeutic effects, and pain relief
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
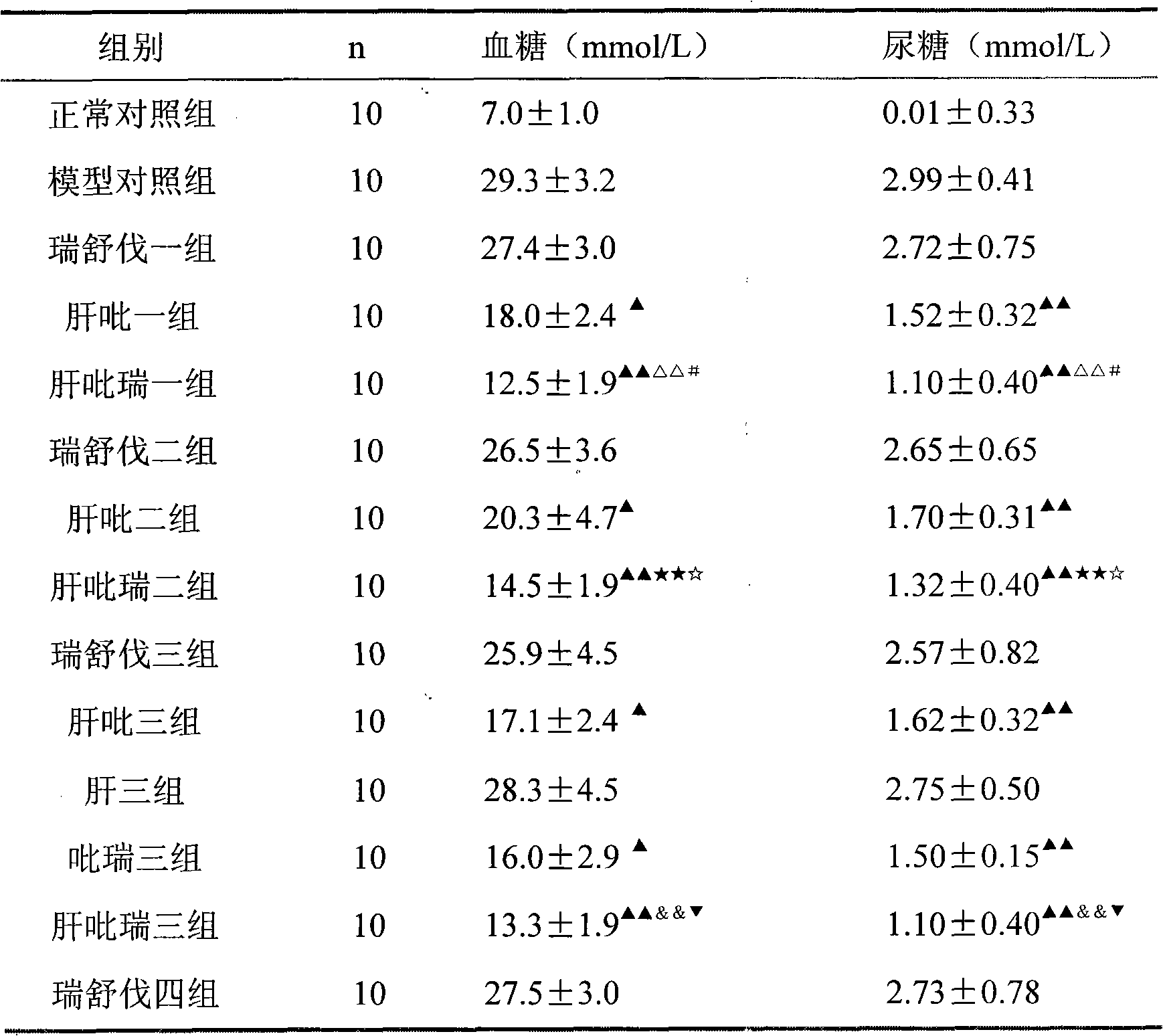
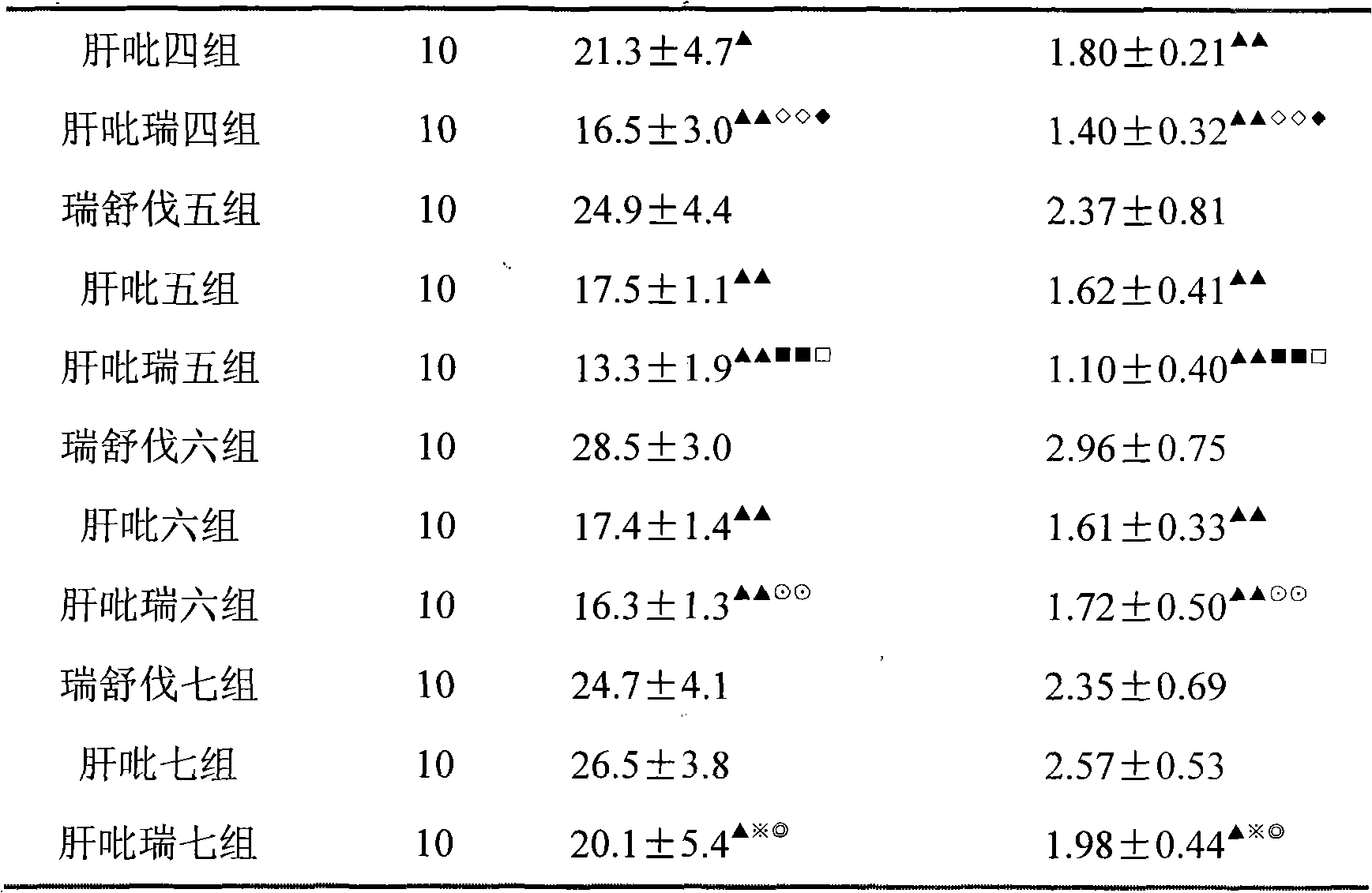
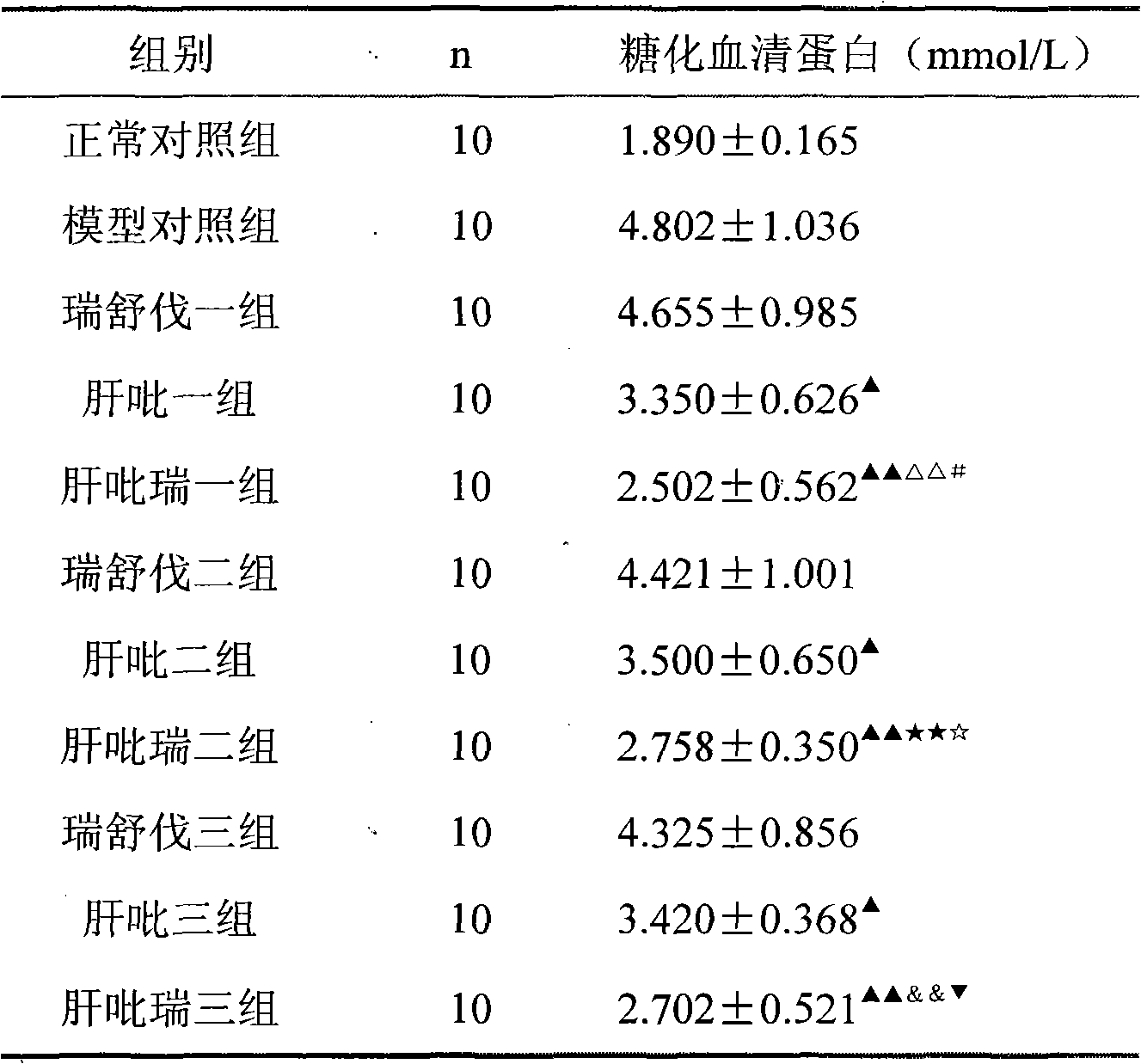
Examples
Embodiment 1
[0033] Example 1 tablet
[0034] Prescription rosuvastatin calcium 1g (calculated as rosuvastatin, the same below)
[0035] Low-molecular-weight heparin sodium 5g (calculated as low-molecular-weight heparin, the same below)
[0036] Pioglitazone hydrochloride 120g (calculated as pioglitazone, the same below)
[0037] Microcrystalline Cellulose 1000g
[0038] 2% hydroxypropyl methylcellulose solution appropriate amount
[0039] Low-substituted hydroxypropyl cellulose 80g
[0041] Preparation process: Rosuvastatin calcium, low-molecular-weight heparin sodium and pioglitazone hydrochloride passed through a 100-mesh sieve, microcrystalline cellulose and low-substituted hydroxypropyl cellulose passed through a 80-mesh sieve, and the prescribed amount of rosuvastatin calcium, low-molecular-weight hydroxypropyl cellulose was weighed. Molecular heparin sodium and pioglitazone hydrochloride are mixed evenly with low-substituted hyd...
Embodiment 2
[0042] Example 2 tablet
[0043] Prescription Rosuvastatin Calcium 1g
[0044] Low molecular weight heparin sodium 1000g
[0045] Pioglitazone Hydrochloride 3g
[0046] Microcrystalline Cellulose 2000g
[0047] 2% hydroxypropyl methylcellulose solution appropriate amount
[0048] Low-substituted hydroxypropyl cellulose 700g
[0050] The preparation process is the same as in Example 1.
Embodiment 3
[0051] Example 3 tablet
[0052] Prescription Rosuvastatin Calcium 1g
[0053] Low molecular weight heparin sodium 1000g
[0054] Pioglitazone Hydrochloride 120g
[0055] Microcrystalline Cellulose 2000g
[0056] 2% hydroxypropyl methylcellulose solution appropriate amount
[0057] Low-substituted hydroxypropyl cellulose 900g
[0059] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com