Diterpenoid compounds with antifouling activities
A compound and diterpenoid technology, applied in the field of diterpenoids, can solve the problems of no anti-fouling active diterpenoids, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
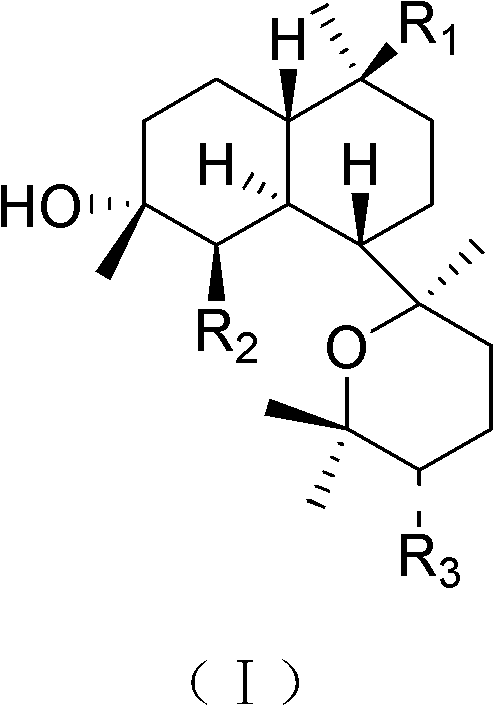
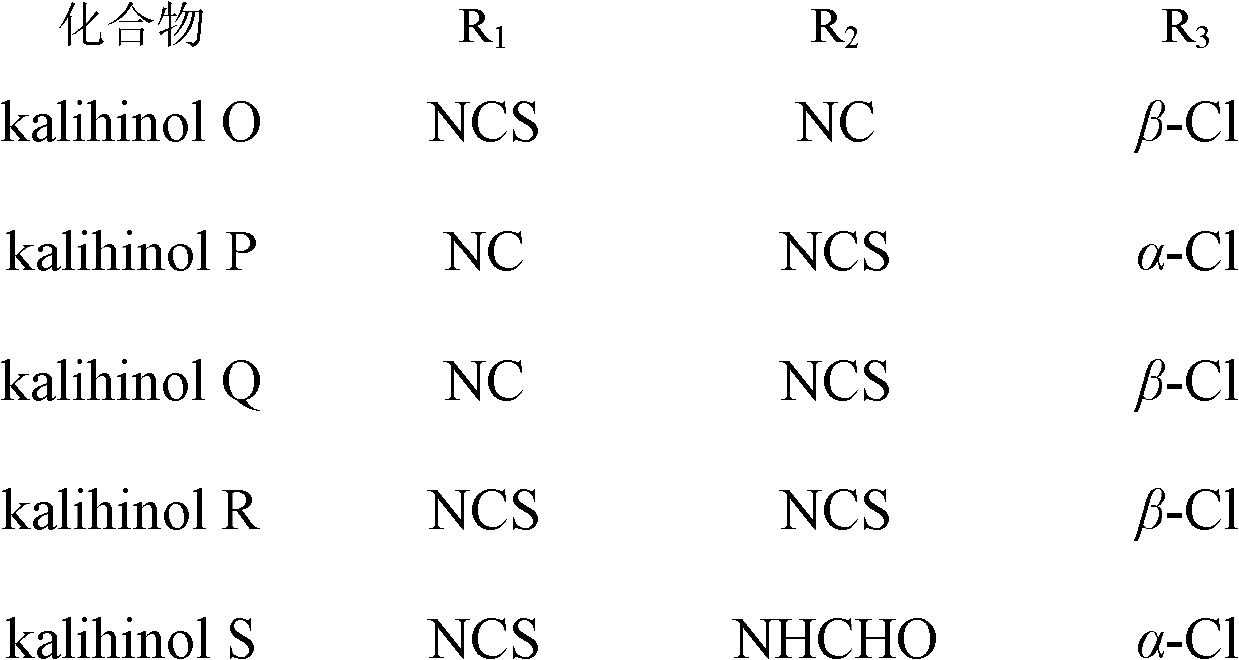
[0021] Embodiment 1. prepare diterpenoid kalihinol O~S of the present invention
[0022] 1. Preparation of Total Extract
[0023] Take the wet weight of sponge A.cavernosa 5.5 kg, use 10 times the volume of acetone solution to leak and extract, combine the extracts, recover the solvent under reduced pressure, and obtain the crude extract;
[0024] 2. Separation and purification
[0025] The crude extract was dissolved in water, extracted three times with dichloromethane, the extract was dispersed in 60%-90% methanol and extracted three times with petroleum ether and dichloromethane successively, and the solvent was recovered under reduced pressure to obtain the petroleum ether fraction (49g) and Dichloromethane fraction (30g) extract; put the dichloromethane extract on silica gel decompression column chromatography as usual, use petroleum ether-acetone system at 100:1-50:1-30:1-15:1-10: 1-5:1-1:1 gradient elution was carried out, and 14 partial eluents Fr1~Fr14 were obtained...
Embodiment 2
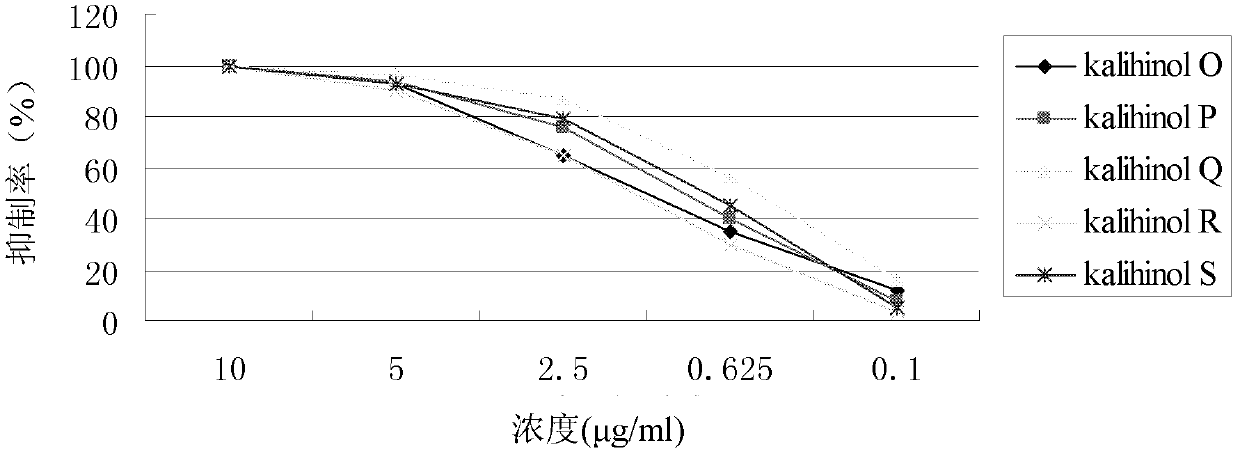
[0036] Embodiment 2: Antifouling activity test of the compound of the present invention
[0037] The most harmful fouling organism barnacle B. amphitrite larvae were selected to establish the anti-fouling activity screening model. Adult barnacles were collected from the intertidal zone of Hong Kong (22°19′N, 114°16′E) and fed on Chaetoceros gracilis, and cultured from nauplii to Venus larvae in 0.22-μm-filtered seawater stage, the culture temperature is 28°C, and the anti-fouling activity of Venus stage larvae is tested.
[0038] The test compounds kalihinol O~P were dissolved in dimethyl sulfoxide to form a series of concentration gradients (0.5~10 μg / ml), and sterile filtered seawater was used as the control group. Take about 20 barnacle larvae and put them into a 24-well polystyrene plate, add 1ml of test solution to each well, and make 5 replicate samples for each concentration, and place the 24-well cell culture plate in a 30°C incubator for 24 hours. Observe the number...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com