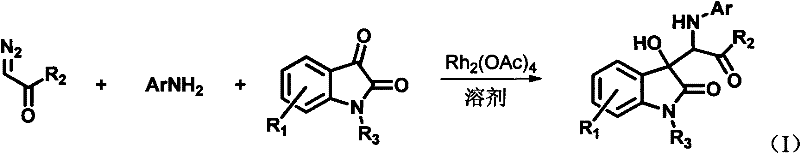
3-substituted-3-hydroxyindazolone derivatives, and preparation method and application thereof
A technology for hydroxyindolinone and derivatives, which is applied in the field of 3-substituted-3-hydroxyindolinone derivatives and their preparation, can solve the problems of many reaction steps, complicated operations and the like, and achieves high selectivity, simple and safe operation , the effect of inhibiting cell growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
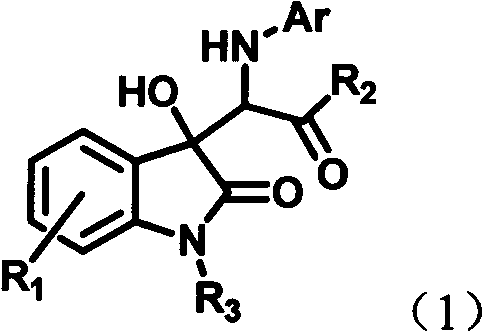
[0055] Dissolve 6-chloroisatin (0.28mmol), aniline (0.42mmol) and rhodium acetate (0.0028mmol) in 4mL of tetrahydrofuran to form a reaction system, and dissolve diazoacetanilide (0.42mmol) in 2mL of tetrahydrofuran to form a solution. The solution of diazoacetanilide dissolved in tetrahydrofuran was added dropwise into the reaction system within 1 hour using an automatic injection pump. After the sample injection was completed, the stirring reaction was continued at room temperature for 1 hour. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, the structure of which was shown in formula (1-1). The crude product was purified by column chromatography (petroleum ether:ethyl acetate=5:1-2:1) to obtain a pure product. Yield: 84%, dr value: 32:68.
[0056] 1 H-NMR (DMSO-d 6 , 400MHz): δ10.47(s, 1H), 9.65(s, 1H), 6.62-7.69(m, 14H), 5.73(d, 1H), 4.37(s, 1H).;
[0057] 13 C-NMR (DMSO-d 6 , 125MHz): δ177.2, 167.6, 147.9, 1...
Embodiment 2
[0059]
[0060] Dissolve 6-chloroisatin (0.28mmol), aniline (0.42mmol) and rhodium acetate (0.0028mmol) in 4mL tetrahydrofuran to form a reaction system, and dissolve diazoacetyl-p-methoxyaniline (0.42mmol) in 2mL tetrahydrofuran A solution was formed, and at normal temperature, a solution of diazoacetanilide dissolved in tetrahydrofuran was added dropwise to the reaction system within 1 hour by using an auto-sampling pump. After the sample injection was completed, the stirring reaction was continued at room temperature for 1 hour. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, the structure of which was shown in formula (1-2). The crude product was purified by column chromatography (petroleum ether:ethyl acetate=5:1-2:1) to obtain a pure product. Yield: 85%, dr value: 30:70.
[0061] 1 H-NMR (DMSO-d 6 , 400MHz): δ10.49(s, 1H), 10.15(s, 1H), 6.47-7.55(m, 12H), 6.47(s, 1H), 4.70(d, 1H), 4.34(d, 1H), 3.71(s, 3H).;
[0062...
Embodiment 3
[0064]
[0065] 6-chloroisatin (0.28mmol), aniline (0.42mmol) and rhodium acetate (0.0028mmol) were dissolved in 4mL tetrahydrofuran to form a reaction system, diazoacetyl n-propylamine (0.42mmol) was dissolved in 2mL tetrahydrofuran to form a solution, At normal temperature, a solution of diazoacetanilide dissolved in tetrahydrofuran was added dropwise into the reaction system within 1 hour by using an automatic injection pump. After the sample injection was completed, the stirring reaction was continued at room temperature for 1 hour. The solvent was removed by rotary evaporation under reduced pressure to obtain a crude product, the structure of which was shown in formula (1-3). The crude product was purified by column chromatography (petroleum ether:ethyl acetate=5:1-2:1) to obtain a pure product. Yield: 66%, dr value: 27:73.
[0066] 1 H-NMR (DMSO-d 6 , 400MHz): δ10.43(s, 1H), 8.10(s, 1H), 6.58-7.35(m, 8H), 6.35(s, 1H), 4.73(d, 1H), 4.41(d, 1H), 2.89-3.09(m, 2H), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com