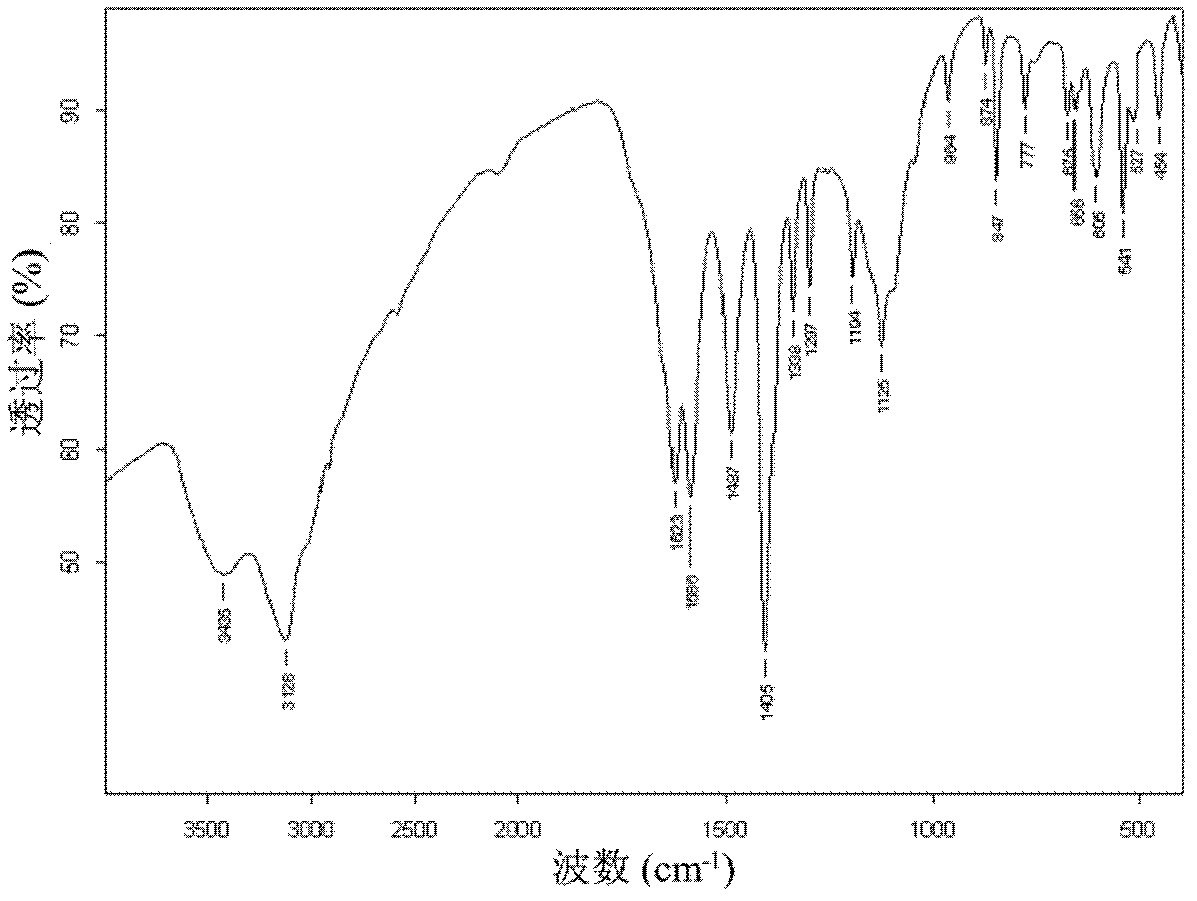
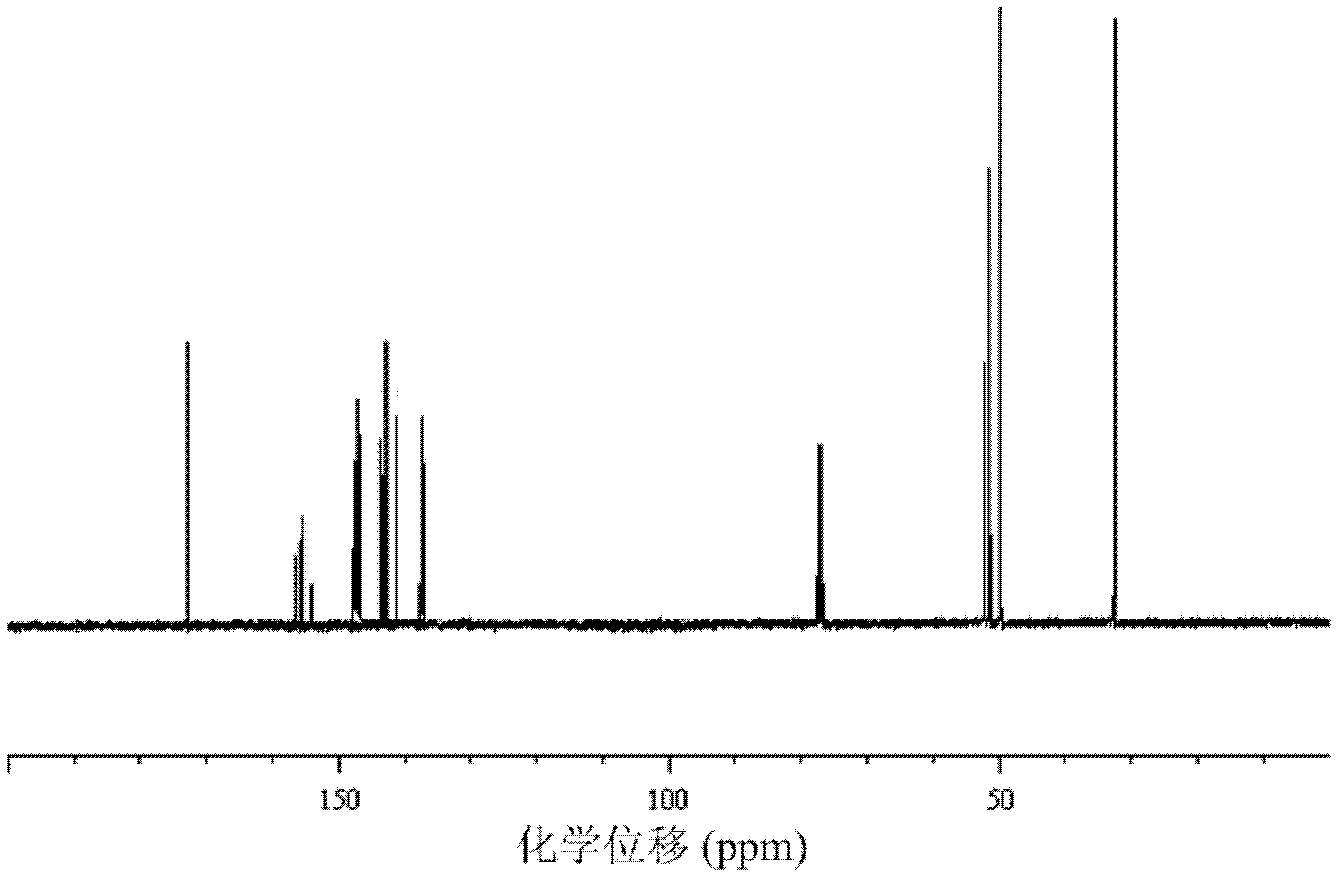
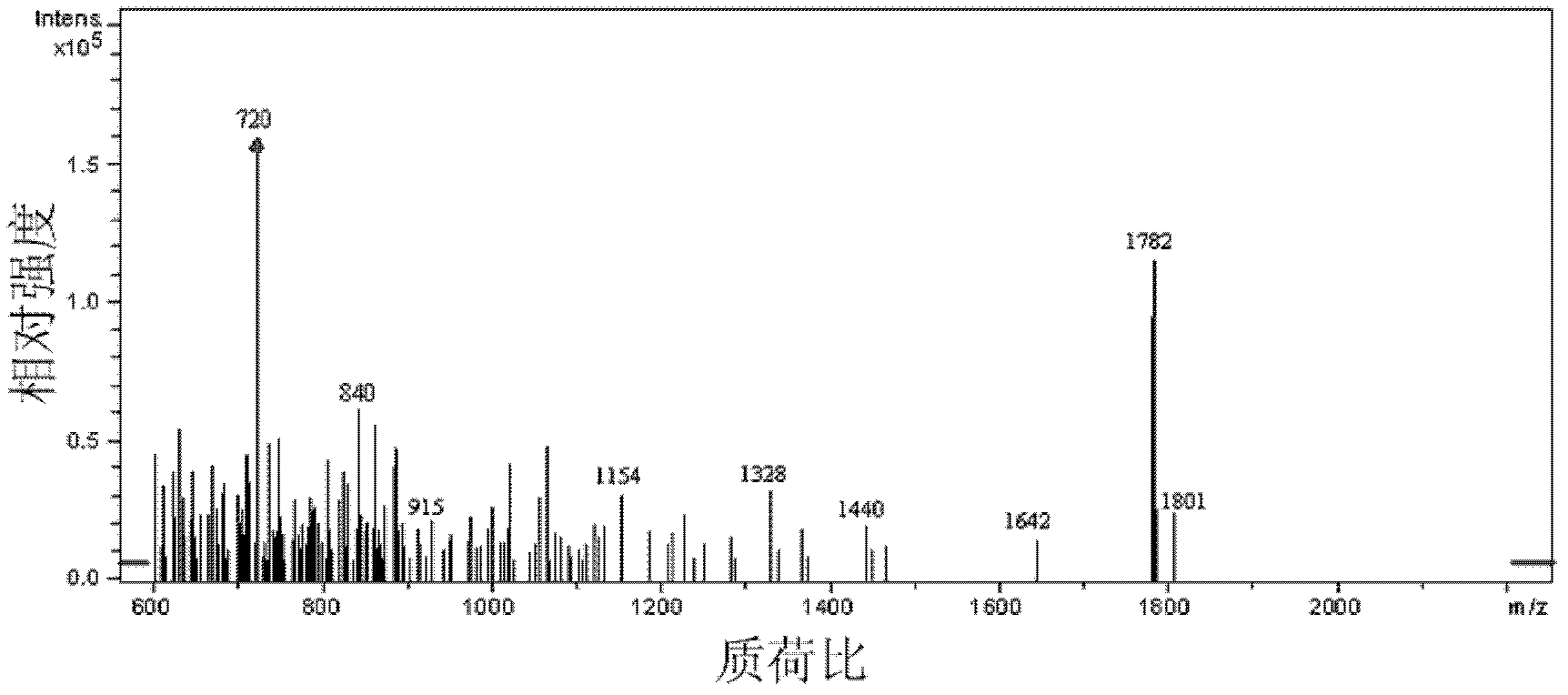
Preparation method for fullerene amino acid derivative
A technology of fullerene amino acids and derivatives, applied in the fields of drug combination, antidote, organic chemistry, etc., can solve the problems of low biological activity and limited amino acids, and achieve the effect of high biological activity and improved reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0009] Embodiment 1: This embodiment is a method for preparing a fullerene amino acid derivative, which is specifically completed according to the following steps:
[0010] 1. Dissolve amino acid and phase transfer catalyst in deionized water at room temperature, and mix well, then add alkaline bromine water, and mix well at room temperature to obtain the mixture; Add the toluene solution of fullerene to the mixture prepared in step 1 at room temperature at 1000 rpm, and then react at 30°C to 80°C under nitrogen protection for 1 to 3 days to obtain the reaction product; 3. First, use toluene to extract Remove the remaining fullerenes in the reaction product, then use gel chromatography to elute, and collect the eluate within 3min to 30min, and finally concentrate and dry the obtained eluent at a temperature of 60°C to 100°C for 12h ~24h, the fullerene amino acid derivatives are obtained.
[0011] The molar ratio of the amino acid to the phase transfer catalyst described in st...
specific Embodiment approach 2
[0040] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the alkaline bromine water described in step one is made of H 2 O, NaOH and Br 2 mixed, and H 2 The molar ratio of O to NaOH is 1: (0.01~1), H 2 O and Br 2 The molar ratio is 1: (0.01~1). Others are the same as in the first embodiment.
specific Embodiment approach 3
[0041] Embodiment 3: The difference between this embodiment and Embodiment 1 or 2 is that the phase transfer catalyst described in step 1 is tetrabutylammonium bromide or tetrabutylammonium hydroxide. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com