Preparation methods of ibuprofen arginine powder injection for injection
A technology for arginine and injection, which is applied in the field of preparation of arginine ibuprofen powder injection, can solve the problems of complex ibuprofen preparation method, high cost, and difficult quality control, and achieve easy quality control and low cost Low, high product yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
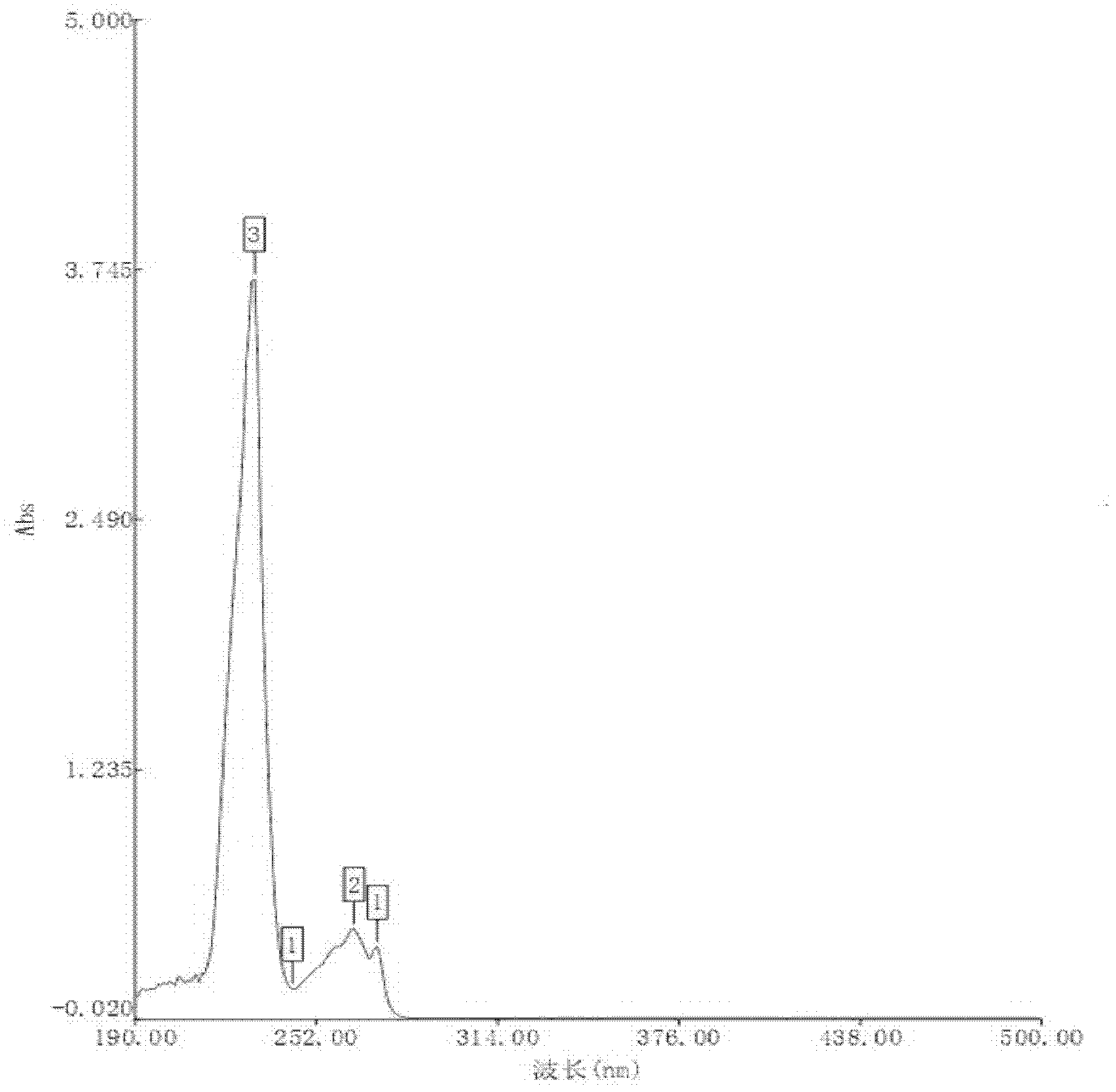
[0010] Specific embodiment 1: The preparation method of arginine ibuprofen powder injection for injection of this embodiment is carried out according to the following steps: 1. Dissolve: mix ibuprofen and arginine at a molar ratio of 1:0.8 to 1.2, and then Add it to purified water at 20~50℃ and stir until it is completely dissolved to obtain the solution; 2. Ultrafiltration: The solution is subjected to ultrafiltration in a sterile environment, and then sterile packaging is carried out to divide the packaged solution; 3. Freezing Drying: freeze-dry the aliquoted solution for 40 to 45 hours to obtain a white freeze-dried powder, which is the arginine ibuprofen powder injection; the mass ratio of purified water to ibuprofen in step 1 is 10: 1.
specific Embodiment approach 2
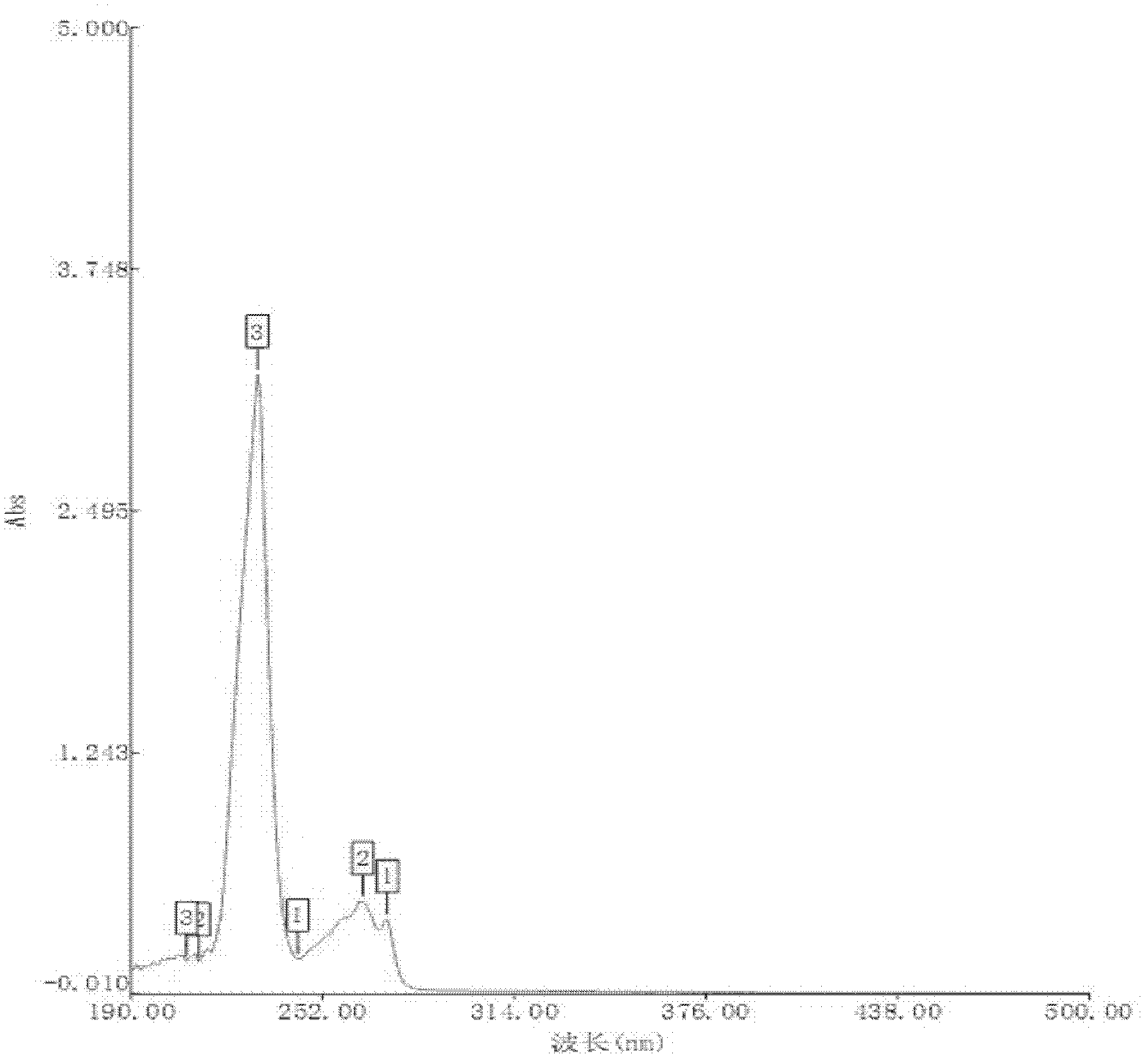
[0011] Specific embodiment two: this embodiment is different from specific embodiment one in that in step one, ibuprofen and arginine are mixed at a molar ratio of 1:1. Others are the same as the first embodiment.
specific Embodiment approach 3
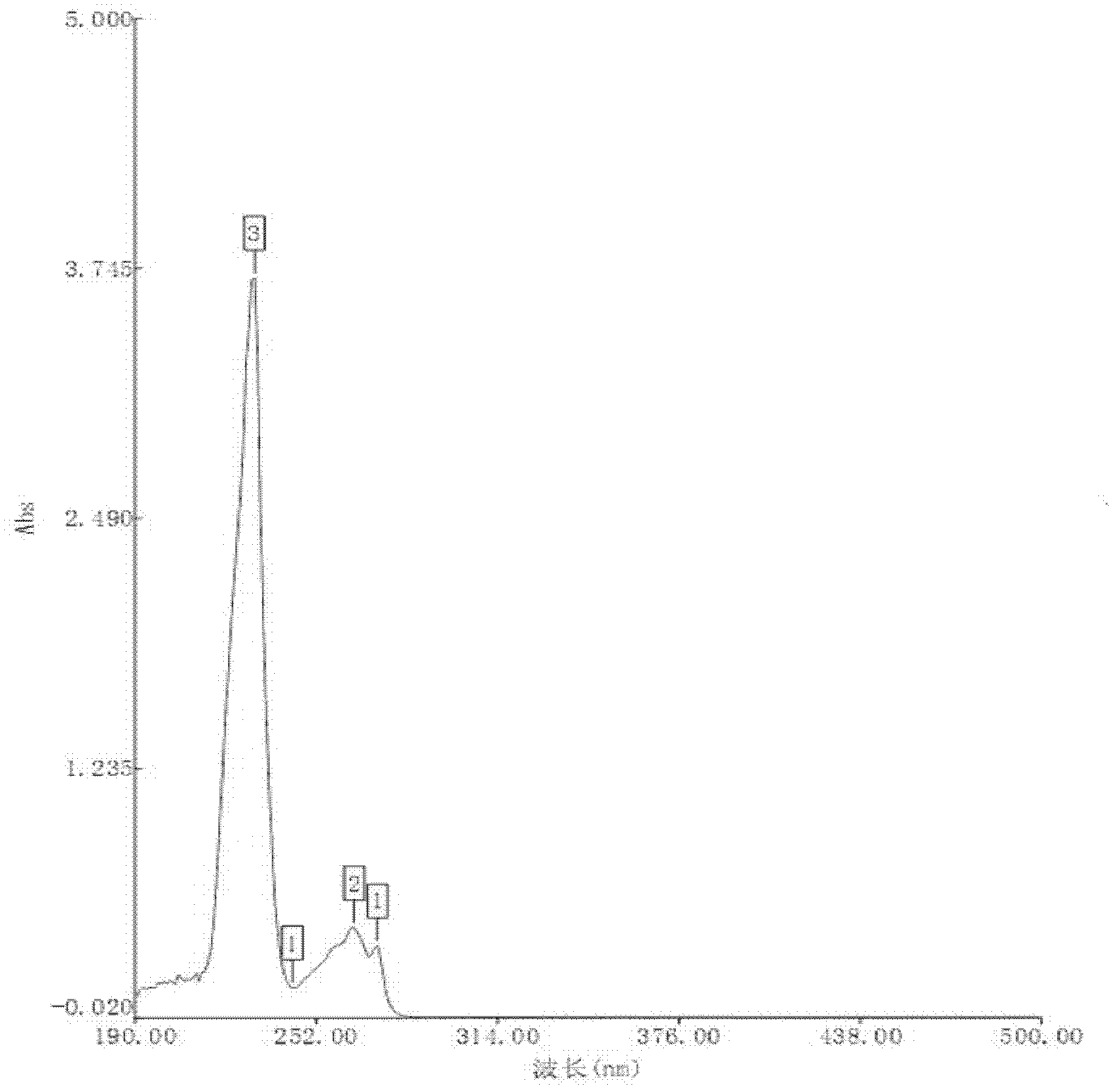
[0012] Specific embodiment three: This embodiment is different from specific embodiment one or two in that in step one, it is added to purified water at 30-40° C. and stirred until completely dissolved. Others are the same as the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com