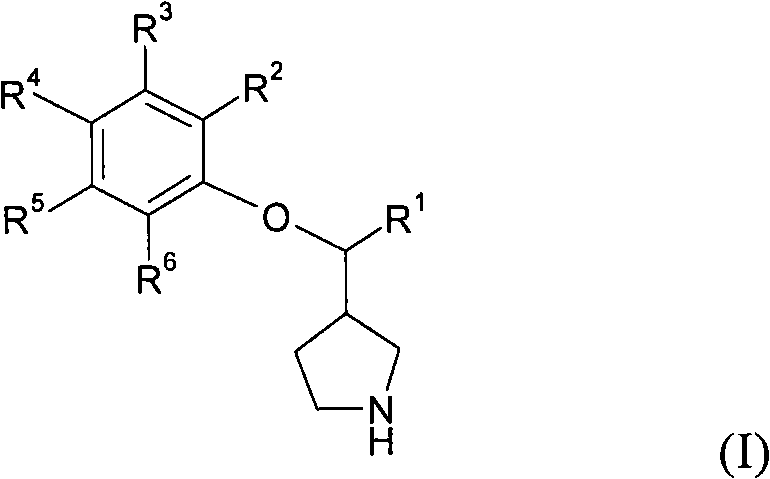
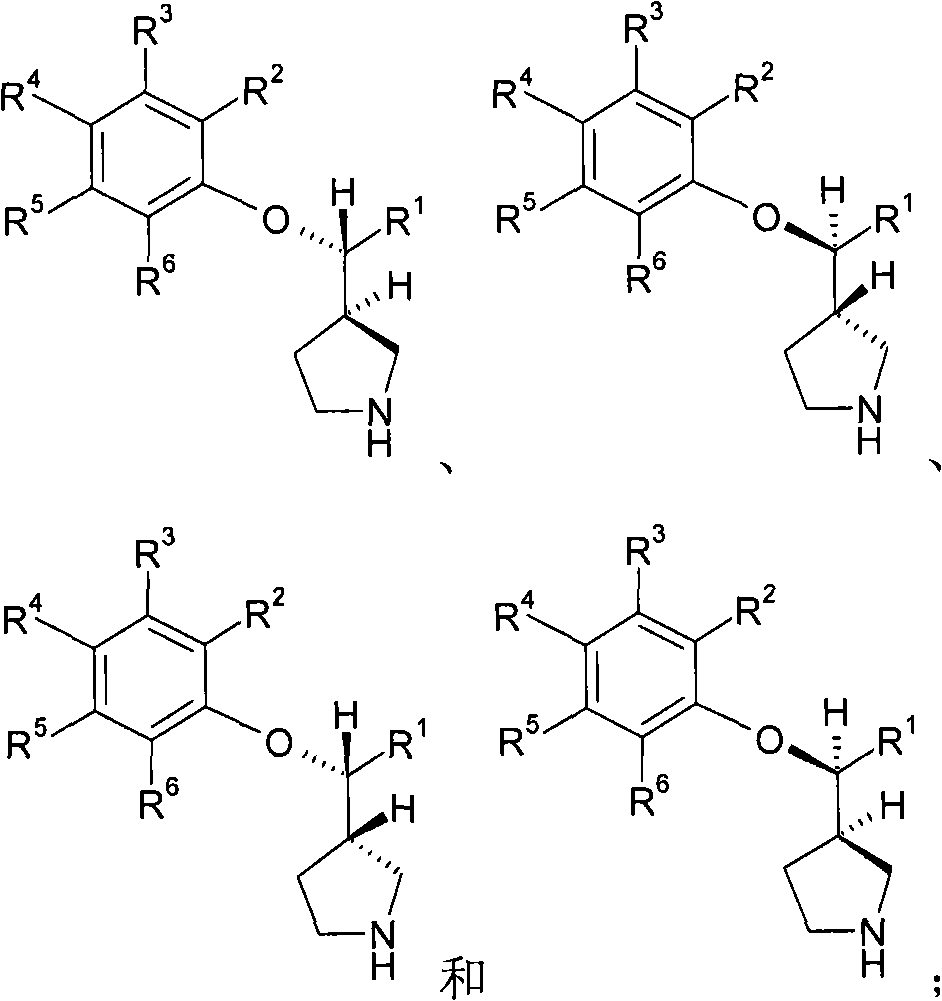
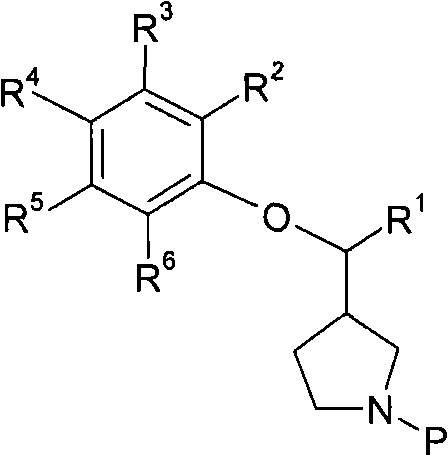
3-phenoxymethylpyrrolidine compounds
A compound, pyrrolidine technology, used in pain conditions and other ailments to address issues such as limiting efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0217] The following Preparations and Examples are provided to illustrate specific embodiments of the invention. However, unless expressly stated otherwise, these specific examples are not intended to limit the scope of the invention in any way.
[0218] Unless otherwise stated, the following abbreviations have the following meanings, and any other abbreviations used herein that are not defined have their standard meanings:
[0219]
[0220]
[0221] Any other abbreviations used herein and not defined have their generally accepted standard meanings. Unless otherwise noted, all materials such as reagents, starting materials, and solvents were purchased from commercial suppliers (e.g., Sigma-Aldrich, Fluka Riedel-de ), etc.) and were used without further purification.
[0222] In all compounds illustrated in the examples, two chiral centers are indicated by * and ** symbols. When elaborating stereochemistry, the carbon atom indicated by the * symbol is assigned first. ...
example 1
[0231] (S)-3-[(S)-1-(2,4-dichlorophenoxy)propyl]pyrrolidine
[0232]
[0233] 60% NaH in mineral oil (60:40, NaH:mineral oil, 10 mg, 260 μmol) was slowly added to (S)-3-((S)-1 in DMF (680 μL, 8.7 mmol) -Hydroxypropyl)pyrrolidine-1-carboxylic acid tert-butyl ester (50mg, 0.2mmol, 1 equiv). The resulting mixture was stirred at room temperature for 15 minutes. 2,4-Dichloro-1-fluorobenzene (76 μL, 3 equiv) was added and the mixture was heated at 90° C. for 3 hours. MeOH (1 mL) was used to stop the reaction. DMF and MeOH were removed under reduced pressure to obtain the BOC-protected intermediate (S)-3-[(S)-1-(2,4-dichlorophenoxy)propyl]pyrrolidine-1-carboxylic acid tert butyl ester. The protecting group was removed using 1.25M HCl in EtOH (1.7 mL, 2.2 mmol). The mixture was stirred overnight at room temperature. The product was then purified by preparative HPLC to afford the title compound (41.5 mg) as the mono-TFA salt. MS m / z: C 13 h 17 Cl 2 [M+H] of NO + The cal...
example 2
[0237] Compounds 2-1 to 2-75 of formula IIa were prepared as mono-TFA salts according to the procedures set forth in the examples above and substituting appropriate starting materials and reagents:
[0238]
[0239]
[0240]
[0241]
[0242] 1 (S)-3-[(S)-1-(3-chlorophenoxy)propyl]pyrrolidine
[0243] 2 (S)-3-[(S)-1-(4-chlorophenoxy)propyl]pyrrolidine
[0244] 3. (R)-3-[(R)-1-(4-chlorophenoxy)propyl]pyrrolidine
[0245] 4. (S)-3-[(R)-1-(4-chlorophenoxy)propyl]pyrrolidine
[0246] 5. (S)-3-[(S)-1-(4-chloro-3-fluorophenoxy)propyl]pyrrolidine
[0247] 6. (S)-3-[(R)-1-(4-chloro-3-fluorophenoxy)propyl]pyrrolidine
[0248] 7. (S)-3-[(S)-1-(3,4-dichlorophenoxy)propyl]pyrrolidine
[0249] 8. (S)-3-[(R)-1-(3,4-dichlorophenoxy)propyl]pyrrolidine
[0250] 9. (S)-3-[(S)-1-(3-fluorophenoxy)propyl]pyrrolidine
[0251] 10. (S)-3-[(S)-1-(3-chloro-5-fluorophenoxy)propyl]pyrrolidine
[0252] 11. (S)-3-[(R)-1-(3-chloro-5-fluorophenoxy)propyl]pyrrolidine
[0253] 12. (S)-3-[(S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com