Catalyst used in alkylation reaction, preparation method and application of catalyst
A technology for an alkylation reaction and a catalyst is applied in the field of an alkylation reaction catalyst and its preparation to achieve the effects of high crystallinity, improved activity stability and reduced total synthesis time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Tetraethylammonium hydroxide (TEAOH) is used as template, and the raw materials are borax, sodium hydroxide and silica gel.
[0037] The feeding ratio is:
[0038] SiO 2 / B 2 o 3 =30;TEAOH / B 2 o 3 =3.6; Al 2 o 3 / B 2 o 3 =0
[0039] Na 2 O / B 2 o 3 = 2; H 2 O / B 2 o 3 =200.
[0040] The first stage reaction conditions: 120°C, time: 20 hours
[0041] Second stage reaction conditions: 140°C, time: 40 hours
[0042] The third stage reaction condition: 180°C, time: 8 hours.
[0043] The synthetic sample is A.
Embodiment 2
[0045] Tetraethylammonium hydroxide (TEAOH) is used as a template, and the raw materials are boric acid, sodium hydroxide and water glass.
[0046] The feeding ratio is:
[0047] SiO 2 / B 2 o 3 =55;TEAOH / B 2 o 3 =1.8; Al 2 o 3 / B 2 o 3 =0.5
[0048] Na 2 O / B 2 o 3 =12;H 2 O / B 2 o 3 =400.
[0049] The first stage reaction conditions: 140°C, time: 30 hours
[0050] Second stage reaction conditions: 150°C, time: 48 hours
[0051] The reaction condition of the third stage: 180°C, the time: 20 hours.
[0052] The main properties of the products obtained are listed in Table 1.
[0053] get sample B
[0054] Table 1B-Main physicochemical properties of β products
[0055] sample
Embodiment 3
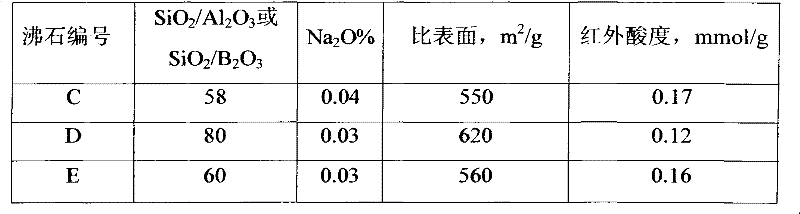
[0057] The invention synthesizes modified B-beta zeolite C. Take 2000ml of A sample slurry, containing 400g of solid phase (on a dry basis), dilute the solid-liquid volume ratio to 1:10 with clean water, then add ammonium nitrate to make the concentration of ammonium nitrate in the solution 2.0M, stir, and heat up to 90 Stir at ~95°C for 2 hours at a constant temperature, then cool down to 5-60°C for filtration, and perform a second exchange of the wet filter cake under the same conditions as the first. The B-beta zeolite that has been exchanged twice with ammonium salts is washed until the pH reaches 5-6, and then placed in a drying oven and dried at 110-120° C. for 6 hours (dry basis is 88% by weight). The dried B-β zeolite is placed in a muffle furnace and rapidly raised to 250°C, kept at a constant temperature for 2 hours, then rapidly raised to 400°C, kept at a constant temperature for 4 hours, and finally raised to 540°C, kept at a constant temperature for 10 hours. Aft...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com