On-line integrity test method of filter
A test method and integrity detection technology, applied in chemical instruments and methods, filtration separation, separation methods, etc., can solve problems such as low pressure, and achieve the effect of improving production efficiency, ensuring real-time monitoring, and ensuring accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] In order to make the present invention more obvious and understandable, a preferred embodiment is hereby described in detail as follows in conjunction with the accompanying drawings for the post-SIP integrity detection of the sterilizing filter.
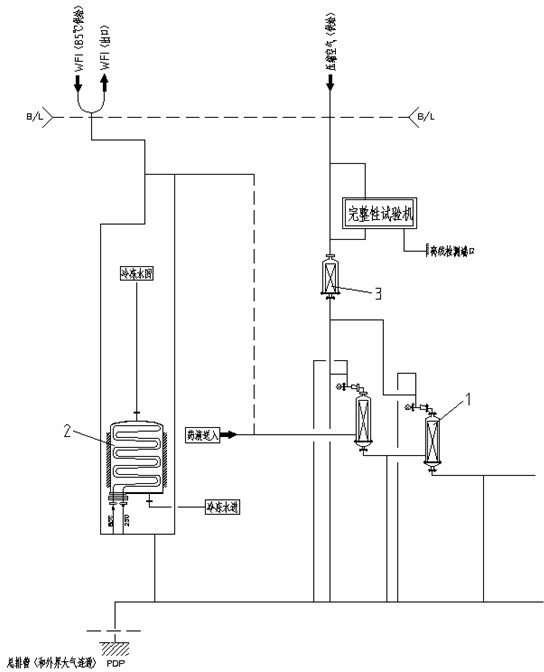
[0025] A filter online integrity testing method provided in this embodiment relies on such as figure 1 The sterilizing filtration system shown, the system includes two cascaded sterilizing filters 1, a heat exchanger 2, an air filter 3 and an integrity testing machine, the liquid medicine is connected through the liquid medicine feeding pipeline All levels of sterilizing filters, the entire integrity detection circuit is connected by WFI pipeline and compressed air pipeline.
[0026] combine figure 1 , an online integrity testing method after a filter SIP in the present embodiment, the steps are:
[0027] Step 1. Connect the bypass of the integrity testing machine in series on the compressed air pipeline of the sterilizing f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com