Antibodies that recognize sulphatides and sulphated proteoglycans and the use thereof
A proteoglycan, sulfatide technology, applied in the field of monoclonal antibodies, can solve problems such as doubts, failure of phase III clinical trials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
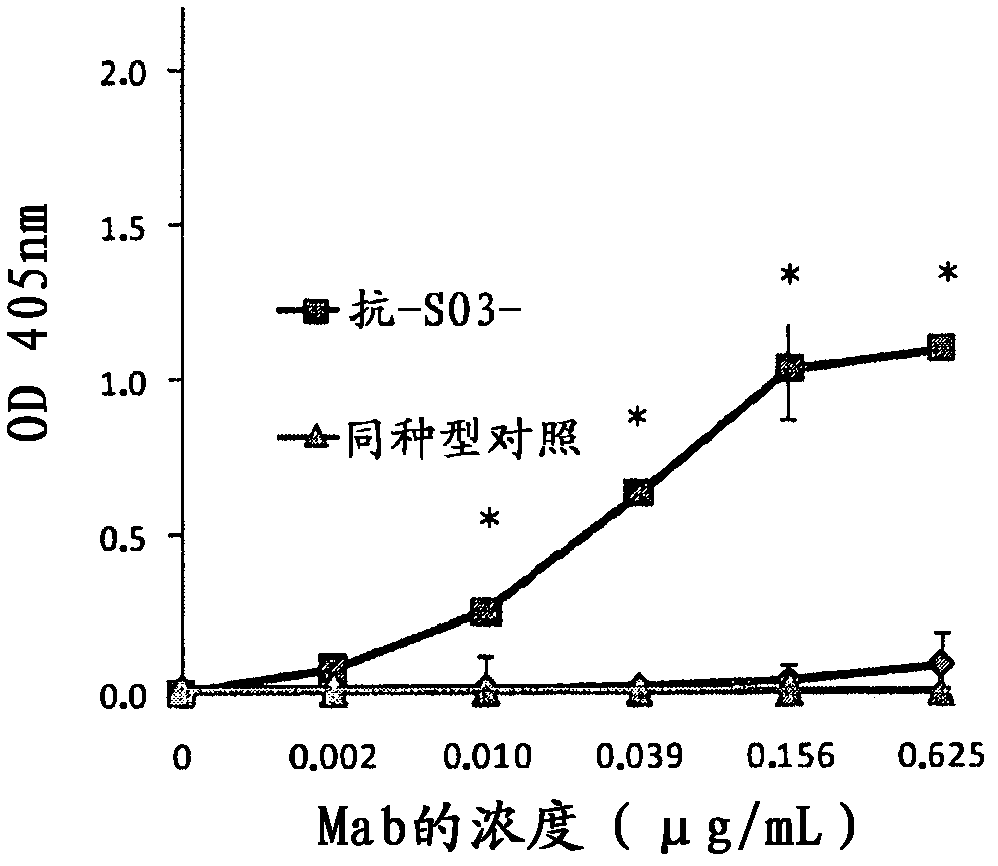
[0093] Example 1. Recognition of chimeric MAb anti-SO3 for bovine sulfatide
[0094] Using ELISA, PolySorp plates, Nunc, were coated with sulfatide solution at a concentration of 4 μg / mL in methanol at 50 μL / well, and the solvent was evaporated by incubation at 37° C. for 90 minutes. Then, the plate was blocked with phosphate buffered saline (PBS) containing 1% bovine serum albumin (SAB) at 200 μL / well at room temperature for 1 hour. Then, 50 μL / well of different concentrations of chimeric antibody anti-SO3 in PBS was added and incubated at 37° C. for 1 hour. Plates were then washed with PBS and 50 μL / well of goat antiserum anti-human gamma chain conjugated to alkaline phosphatase (Sigma) was added. The plates were incubated at 37°C for 1 hour, after which the plates were washed again and 100 μL / well of substrate solution consisting of 1 mg / mL p-nitrophenyl phosphate in diethanolamine buffer pH 9.8 was added. After incubation for 30 minutes at room temperature, the absorbanc...
Embodiment 2
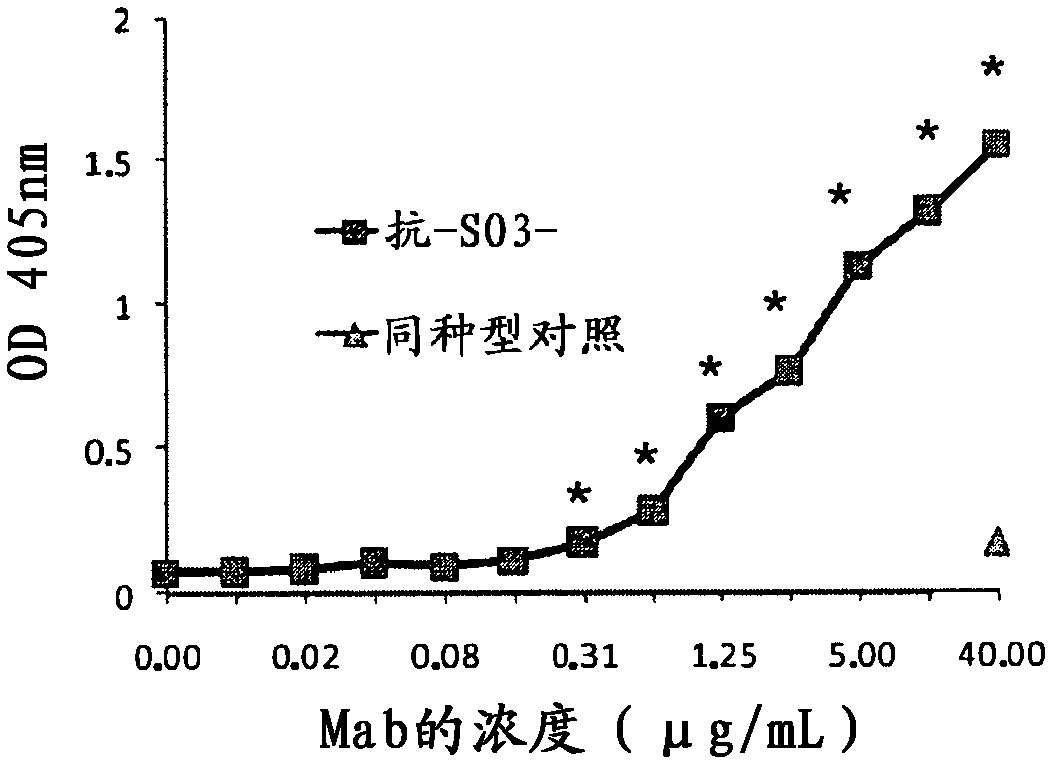
[0096] Example 2. Heparin recognition test
[0097] It was then determined whether the chimeric monoclonal anti-SO3 recognized sulfated molecules more complex than sulfatides. Heparin, a highly sulfated molecule used as a model for sulfated glycosaminoglycans, was chosen for this study.
[0098] The test for anti-heparin reactivity was performed based on the ELISA technique for biglican developed by Skalen, K.M.y cols (Nature 417:750-754, 2002) with slight modifications. Maxisorp microtiter plates (Nunc) were coated with 10 μg / mL (100 μL / well) of heparin (Sigma) in Hepes buffered saline solution (HBSS) (20 mM Hepes, 150 mM NaCl, pH 7.4) and incubated overnight at 4°C . Plates were washed 3 times with HBSS and then blocked with HBSS containing 1% SAB (HBSS-BSA) for 1 hour at room temperature. Wash the plate 3 times with 0.02% HBSS-Tween20 (HBSS-T), and add serial dilutions of chimeric monoclonal anti-SO3 within 1 hour at room temperature: from binding buffer (10mM Hepes, 20m...
Embodiment 3
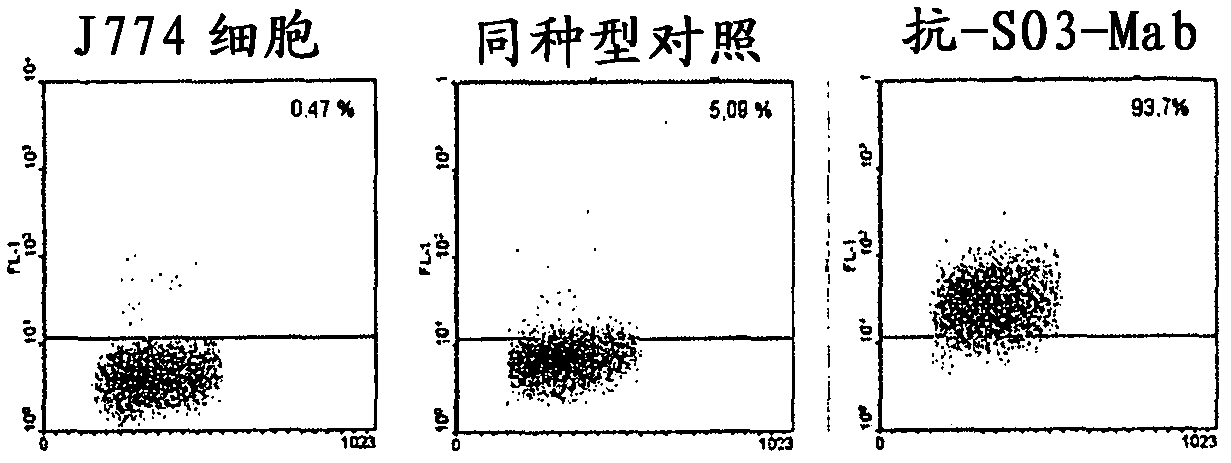
[0100] Example 3: Determination of recognition of the J774 cell line by flow cytometry
[0101] Monocytes and macrophages are important in inflammatory processes such as atherosclerosis ( B E. Physiol Rev 83:1069-1112, 2003). These cells synthesize proteoglycans, and it has been demonstrated that certain pathways for the incorporation of oxidized LDL in macrophages involve cell membrane proteoglycans in the formation of foam cells (Halvorsen B, et al. Biochem J. 331:743--752, 1998).
[0102] To determine whether anti-SO3-chimeric antibodies could recognize macrophages, we performed flow cytometry experiments using the murine macrophage cell line J774 cultured in cells supplemented with 8% inactivated fetal calf serum (SFT; Gibco), 2 mM L-glutamine, 100 U / mL penicillin, 100 μg / mL streptomycin in DMEM-F12 (Gibco BRL, Paisley, Scotland).
[0103] Cells (0.5x10 6 / tube) was incubated with 20 μL / tube of inactivated rabbit serum at 37° C. for 10 minutes to block Fc-γ receptor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com