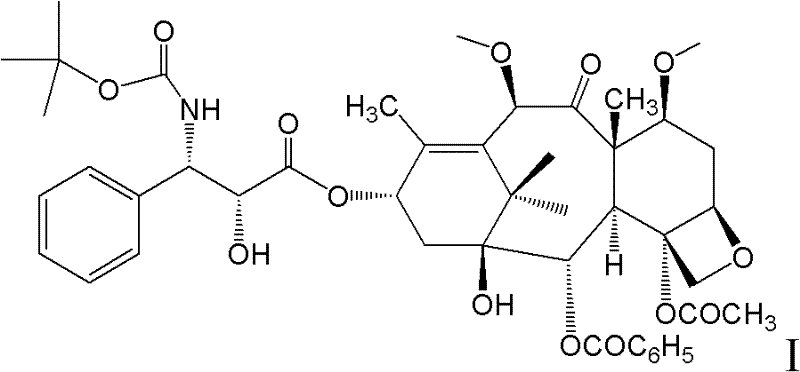
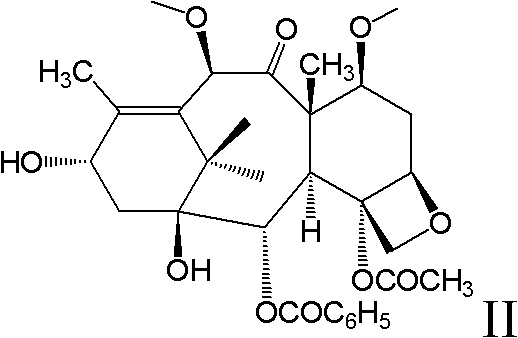
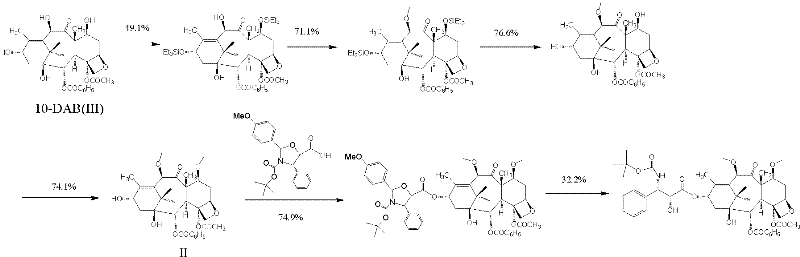
New taxane derivative and preparation method thereof
A compound, the technology of triethylsilyl, is applied in the field of preparing the anti-tumor drug cabazitaxel, which can solve the problems of high cost, low yield, and many steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0084] III. Preparation method of compound IV.
[0085] Compound IV can use compound V as a raw material (see Bioorganic & Medicinal Chemistry Letters, Volume 13, No. 11, 2419-2482.1993 for the synthesis method of compound V), and the trichloroethoxycarbonyl group in compound V can be selectively removed by zinc powder reduction. (Troc) Obtained.
[0086]
[0087] In the formula, R1 is a hydroxyl protecting group or hydrogen.
[0088] In existing literature reports, trichloroethoxycarbonyl (Troc) can be deprotected in the presence of zinc powder and acetic acid as a solvent. In the present invention, when R1 is stable under acetic acid conditions, this condition can be used to selectively remove the trichloroethoxycarbonyl (Troc), such as R1 is triethylsilyl, tert-butyldimethylsilyl (TBS) and tert-butyldiphenylsilyl. However, due to the presence of strong acid acetic acid, R1 will be removed in a small amount and partly, which will affect the yield of the reaction, and post-treatm...
Embodiment 1
[0121] Add 80ml of acetic acid and 12g of active zinc powder to a 100ml reaction flask, stir evenly, and after heating to 60-70°C, add 5g of 2'-TBS-7, 10-Troc-Docetaxel. After stirring the reaction for 5 hours, TLC showed that the reaction was complete. After the reaction, filter, add 80ml ethyl acetate and 80ml water to the filtrate. After separation, the organic phase is washed with water (50ml), saturated sodium bicarbonate (50ml×2), saturated sodium chloride (50ml), anhydrous sulfuric acid Sodium is dry. After filtering out the anhydrous sodium sulfate, it was concentrated to dryness under reduced pressure to obtain a white solid, namely 2.5 g of 2'-TBS-docetaxel, with a yield of 81%.
[0122] MS (m / z): 972 (M+Na). 1 HNMR (500MHz) δ 0.31 (6H, m), 0.92 (9H, m), 1.13 (3H, s), 1.30 (12H, m), 1.72 (7H, m), 1.92 (4H, m), 2.18 ( 1H, m), 2.35 (1H, m), 2.61 (4H, m), 3.95 (1H, d), 4.21 (3H, m), 4.28 (1H, d), 4.54 (1H, s), 4.97 (1H) , D), 5.21 (1H, s), 5.27 (1H, m), 5.50 (1H, m), 5....
Embodiment 2
[0124] At 35~40℃, add 50ml methanol, 10g active zinc powder, 50ml water and 10g ammonium chloride into a 100ml reaction flask, stir well, add 5g 2'-TES-7, 10-Troc-Docetaxel . After stirring the reaction for 4 hours, TLC showed that the reaction was complete. After the reaction, filter, add 80ml ethyl acetate and 80ml water to the filtrate. After separation, the organic phase is washed with water (50ml), saturated sodium bicarbonate (50ml×2), saturated sodium chloride (50ml), anhydrous sulfuric acid Sodium is dry. After filtering out the anhydrous sodium sulfate, it was concentrated to dryness under reduced pressure to obtain a white solid, namely 2.8 g of 2'-TES-docetaxel, with a yield of 90%.
[0125] MS (m / z): 972 (M+Na). 1 HNMR (500MHz) δ 0.39 (6H, m), 0.78 (9H, m), 1.13 (3H, s), 1.30 (12H, m), 1.72 (7H, m), 1.92 (4H, m), 2.18 ( 1H, m), 2.35 (1H, m), 2.61 (4H, m), 3.95 (1H, d), 4.21 (3H, m), 4.28 (1H, d), 4.54 (1H, s), 4.97 (1H) , D), 5.21 (1H, s), 5.27 (1H, m), 5.50 (1H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com