Mycophenolic acid derivatives Penicacids A, B, C and their application in preparing immunosuppression medicaments
A technology of immunosuppression, mycophenolic acid, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
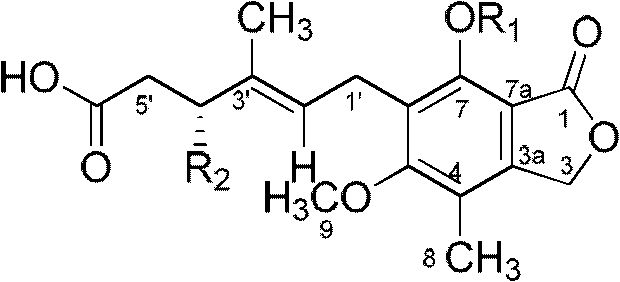
[0019] Preparation and Structure Identification of Penicacids A, B, C
[0020] One, the preparation of Penicacids A, B, C
[0021] 1. Seed culture:
[0022] (1) Preparation of seed medium: 200g of potatoes (peeled), 20g of glucose, 30g of sea salt, add water to make up to 1L, evenly distribute in ten 500mL Erlenmeyer flasks, and sterilize at 121°C for 25 minutes to obtain sterilized seed culture.
[0023] (2) Cultivation of seeds: Inoculate the strain of marine fungus (Penicillium sp.) SOF07 into the above-mentioned seed culture medium, place it on a shaker at a speed of 150 rpm at a temperature of 28° C., and cultivate for 48 hours to obtain a seed culture solution.
[0024] 2. Solid fermentation culture:
[0025] (1) Preparation of fermentation medium: 2000g of rice, 60g of sea salt, and 2000mL of water were evenly distributed in 20 500mL Erlenmeyer flasks, and sterilized at 121°C for 25 minutes to obtain sterilized fermentation medium.
[0026] (2) Fermentation culture:...
Embodiment 2
[0047] The compounds Penicacids A, B and C separated and purified in Example 1 were tested for IMPDH inhibitory activity and mouse splenocyte proliferation inhibitory activity.
[0048] The specific steps are: IMPDH (type II) enzyme inhibitory activity test, adopt the method reported by Magasanik etc. (Magasanik, B.; Moyed, H.; Gehring, L.J.Biol.Chem.1957,226,339-350.), adopt 96 Well plate, IMPDH (type II) enzyme activity was assessed by detecting NADH formation at 340 nm. Penicacids A, B, and C were dissolved in DMSO, then diluted three times, and then added to the mixture to be tested for pre-incubation, and the final concentration of the enzyme was not more than 2% (V / V). Mycophenolic acid was used as a control.
[0049] The mouse spleen cell proliferation inhibitory activity test, the specific operation is: take the spleen of C57BL / 6 mice, obtain a sterile single-cell suspension through nylon membrane filtration, then centrifuge the cells at 1000rpm for 5 minutes, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com