Intra-intestinal nutrient emulsion for tumor patients and preparation method thereof
An enteral nutrition emulsion, a technology for cancer patients, applied in the field of enteral nutrition emulsion, can solve the problems of poor fat utilization and achieve the effect of enhancing the body's resistance and promoting immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
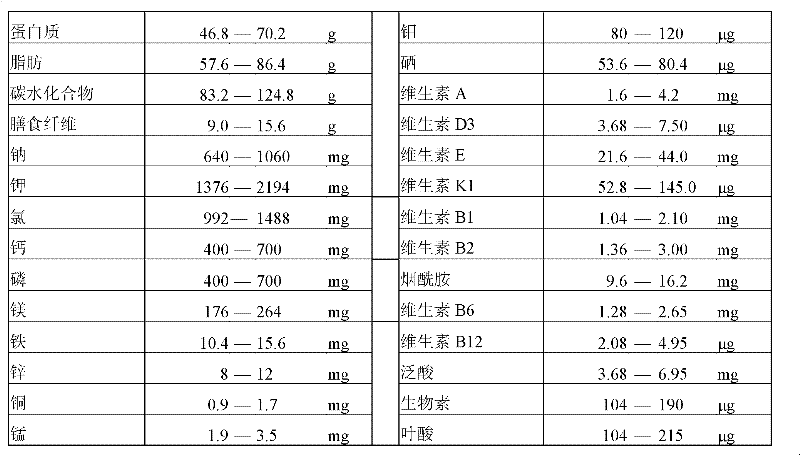
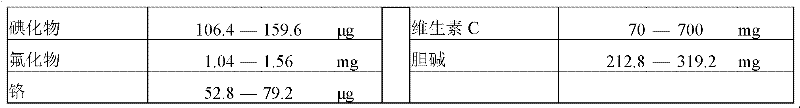
[0039] The feeding formula is shown in Table 3 below.
[0040] Table 3 Example 1 feeding formula.
[0041] Vitamin D3 (100000IU / g)
1.984mg
0.125mg
0.141mg
1.664mg
2.504mg
2.112mg
Vitamin B12
3.280μg
0.653mg
10.800mg
thbrthdrexvbdr
5.040mg
Vitamin K1
0.095mg
glucose
9.425 mg
448mg
Maltodextrin (DE value 11-16)
58.936g
25.6g
sodium caseinate
42.16g
11.2g
5.974g
soybean polysaccharide
7.04g
potassium citrate
1.992g
0.104g
0.208g
0.552g
0.768g
1g ...
Embodiment 2
[0054] The feeding formula is shown in Table 5 below.
[0055] Table 5 embodiment 2 feeding formula
[0056]
[0057]
[0058] The preparation method is the same as in Example 1.
[0059] Table 6 below shows the test results of the product after 0 months and 12 months of airtight storage below 25°C (not frozen).
[0060] Table 6 embodiment 2 product airtight storage after 12 months inspection result
[0061]
[0062]
Embodiment 3
[0064] The feeding formula is shown in Table 7 below.
[0065] Table 7 embodiment 3 feeding formula
[0066]
[0067]
[0068] The preparation method is the same as in Example 1.
[0069] The test results of the products after 0 months and 12 months of airtight storage below 25°C (not frozen) are shown in Table 8 below.
[0070] The inspection result after 12 months of table 8 embodiment 3 product airtight preservation
[0071]
[0072]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com