Quetiapine fumarate sustained-release tablets and preparation method thereof
A technology of quetiapine fumarate and quinoline fumarate, which can be applied to pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the complex preparation process, environmental and human hazards. and irritation, etc., to achieve the effect of simple process and good sustained release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
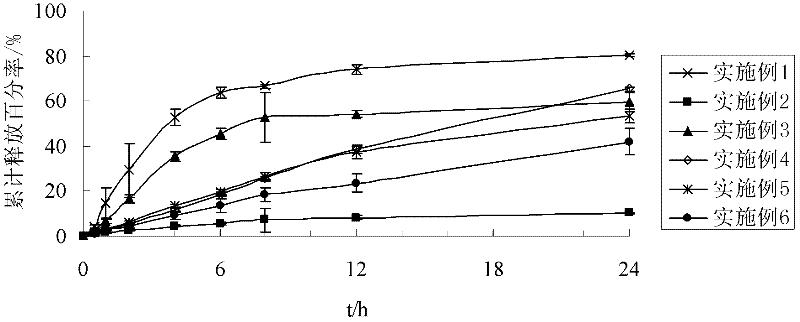
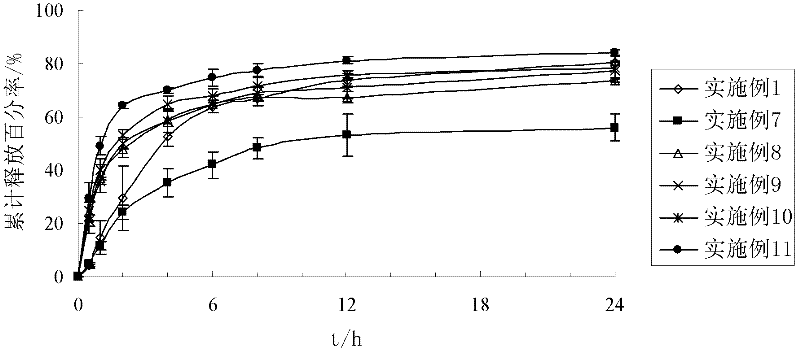
[0032] Example 1: Preparation of Quetiapine Fumarate Sustained Release Tablets
[0033] Take 200g of quetiapine fumarate (calculated as quetiapine) and crush it, pass it through an 80-mesh sieve, and mix it with 58g acrylic resin RL PO, and use 9g 10% polyvinylpyrrolidone K-30 as a soft material as a binder; Granulate with an 18-mesh sieve, dry, use an 18-mesh sieve to sizing, add 3 g of magnesium stearate, mix well, and compress.
Embodiment 2
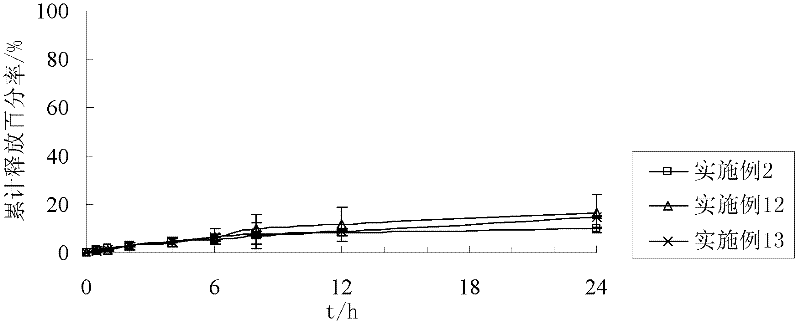
[0034] Example 2: Preparation of Quetiapine Fumarate Sustained Release Tablets
[0035] Take 200g of quetiapine fumarate (calculated as quetiapine) and crush it, pass it through an 80-mesh sieve, and mix it with 58g of acrylic resin RS PO, and use 9g of 10% polyvinylpyrrolidone K-30 as a binder to make a soft material; Granulate with an 18-mesh sieve, dry, use an 18-mesh sieve to sizing, add 3 g of magnesium stearate, mix well, and compress.
Embodiment 3
[0036] Example 3: Preparation of Quetiapine Fumarate Sustained Release Tablets
[0037] Take 200g of quetiapine fumarate (calculated as quetiapine), crush it, pass through an 80-mesh sieve, and mix with 29g acrylic resin RL PO and 29g acrylic resin RS PO, and use 9g 10% polyvinylpyrrolidone K-30 as the adhesive Preparation of soft material; granulate through an 18-mesh sieve, dry, sizing with an 18-mesh sieve, add 3g of magnesium stearate, mix well, and compress.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com