Application of carborane derivatives, nano compound preparation and application of nano compound preparation
A nano-composite and derivative technology, which is applied in the directions of boron compound active ingredients, medical preparations containing active ingredients, inorganic active ingredients, etc. and other problems, to achieve the effect of treating bacterial infection and good antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 The Minimum Inhibitory Concentration Test of Three Carborane Derivatives
[0025] 1.1 Preparation of test drugs: three carborane derivatives shown in structural formulas (1), (2) and (3) were respectively prepared into high-concentration mother solutions with dimethyl sulfoxide, and then diluted with double distilled water The concentration was 1600, 800, 400, 200, 100, 50, 25, 12.5, 6.25μg / mL. Test drugs were stored at -4°C. Before the experiment, the drug was taken out of the 37°C water bath and mixed, shaken well, and the pharmacodynamic experiment was carried out. In the experiment, the final concentration of DMSO should be strictly controlled below 0.5 % (v / v) to ensure that there is no cytotoxicity to the organism.
[0026] 1.2 Experimental strains and culture methods: Standard strains: Staphylococcus aureus (CGMCC1.89), Klebsiella pneumoniae (ATCC700600), Acinetobacter baumannii (ATCC19606), Proteus mirabilis (ATCC12453). Clinical resistant strains...
Embodiment 2
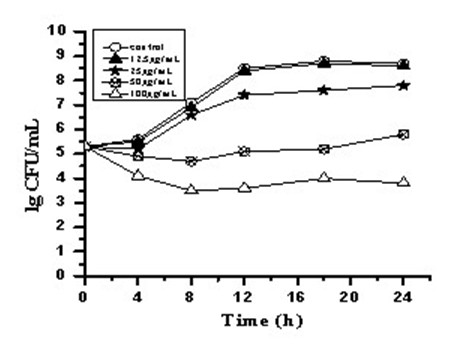
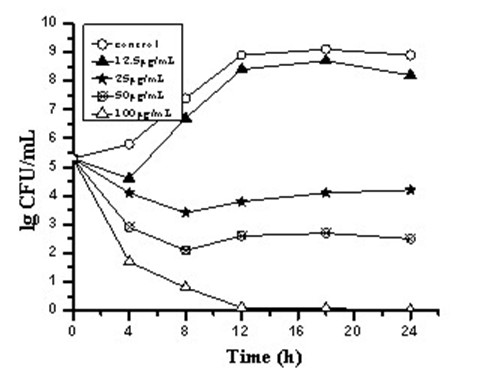
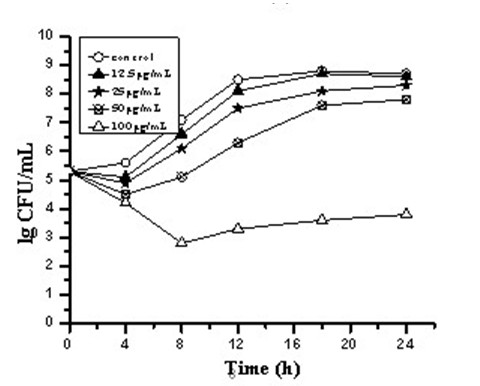
[0029] Example 2 Time-kill curves of three carborane derivative nanocomposite formulations against Staphylococcus aureus, such as figure 1 , 2 , 3.
[0030] Antibacterial characteristics of three carborane derivatives (1), (2), (3) nanocomposite formulations against clinically resistant Staphylococcus aureus strain SA321 were evaluated by time-killing curves. It was tested at different concentrations of 12.5, 25, 50, 100, 200 μg / mL. The bacterial suspension was added with the above-mentioned final concentration of drugs, and the bacterial suspension without drug was set as the control group, and then they were placed in a constant temperature incubator at 37°C for cultivation, and different time intervals were taken at 0, 4, 8, 12, The 16 and 24 h culture solution was counted on the plate colony, and the bactericidal activity was defined as greater than 3 times log compared with the control group 10 Decrease in CFU / mL. The test results showed that the relevant antibac...
Embodiment 3
[0031] Example 3 Comparative test of antibacterial percentage of three carborane derivatives and their nanocomposite preparations
[0032] The cell density is 2 x 10 5 CFU / mL standard strain GCMCC1.89 and bacterial suspension of clinical drug-resistant strain SA321 were added with different final concentrations (8, 16, 32 μg / mL) of compound (1), (2), (3) preparations, not The drug-dosed bacterial suspension was set as the control group, and then placed in a constant temperature incubator at 37°C for 24 hours, and the absorbance value at 600nm was measured with an ultraviolet spectrophotometer. Bacterial inhibition percentage=[1-(OD tested -OD blank ) / (OD control -OD blank )]×100.
[0033] The test results showed that there was no significant difference in the antibacterial percentage of the three carborane derivatives and their nanocomposite preparations against the sensitive and resistant strains of Staphylococcus aureus (P > 0.05). There was no significant differen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com