Method for preparing meptazinol and analogs thereof
A compound and low-level technology, applied in the direction of organic chemistry, etc., can solve the problems of difficult to achieve large-scale production of meptanol, time-consuming post-processing, slow quenching operation, etc., achieve synthesis conditions and process optimization, easy feeding, and easy to overcome fire effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
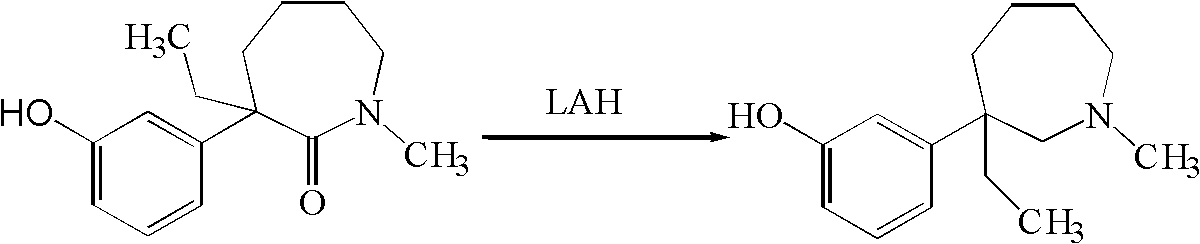
[0046] Example 2 Preparation of 1-methyl-3-ethyl-3-(3-hydroxyphenyl)-hexahydro-1H-azepine (mebutamol) by red aluminum method
[0047] Add 20.0g (0.081mol) 1-methyl-3-ethyl-3-(3-hydroxyphenyl)-hexahydro-1H-azepine-2-one and 400ml methyl For tert-butyl ether, pass nitrogen under stirring and cool down to 10°C with an ice-water bath, add 116.9g (0.405mol, d=1.036) red aluminum dropwise, heat up after dropping, and react at the reflux temperature of methyl tert-butyl ether for 9 After one hour, the reaction solution was cooled to 10°C, and 60.7ml of saturated ammonium chloride solution was slowly added dropwise, and the drop was completed in 10 minutes. The reaction solution was a suspension with a large number of small white particles, and the powder could settle quickly after standing. Filtrate, add 300 ml of methyl tert-butyl ether to the filter cake, stir and wash, filter, combine the filtrates, and distill off the solvent under reduced pressure to obtain 17.8 g of a light yel...
Embodiment 3
[0048] Example 3 Preparation of 1-methyl-3-ethyl-3-(3-hydroxyphenyl)-hexahydro-1H-azepine (mebutamol) by red aluminum method
[0049] Add 20.0g (0.081mol) 1-methyl-3-ethyl-3-(3-hydroxyphenyl)-hexahydro-1H-azepine-2-one and 400ml methyl For tert-butyl ether, pass nitrogen under stirring and cool down to 10°C with an ice-water bath, add 46.7g (0.162mol, d=1.036) red aluminum dropwise, heat up after dropping, and react at the reflux temperature of methyl tert-butyl ether for 30 After one hour, the reaction solution was cooled to 10° C., and 24.3 ml of a saturated ammonium chloride solution was slowly added dropwise, and the dropwise was completed in 10 minutes. Filter, add 300ml of methyl tert-butyl ether to the filter cake, stir and wash, filter, combine the filtrates, evaporate the solvent under reduced pressure to obtain a light yellow crude product, HPLC content: 84.1% of meprotamol, unreacted 1-methyl- 3-Ethyl-3-(3-hydroxyphenyl)-hexahydro-1H-azepin-2-one 15.3%. The crude ...
Embodiment 4 3
[0051] Example 4 Preparation of 1-methyl-3-ethyl-3-(3-hydroxyphenyl)-hexahydro-1H-azepine (mebutamol) by boron trifluoride ether-sodium borohydride method
[0052] Under ice-cooling, add 400ml tetrahydrofuran containing 9.5g (0.243mol) sodium borohydride into a three-necked flask, add 46.0g (0.324mol) boron trifluoride etherate complex with stirring, and stir for 1 hour. Carefully add 20.0g (0.081mol) of 1-methyl-3-ethyl-3-(3-hydroxyphenyl)-hexahydro-1H-azepin-2-one, stir and heat, at the reflux temperature of tetrahydrofuran React for 2 hours. The reaction solution was cooled and neutralized with saturated sodium bicarbonate. The solvent was removed under reduced pressure, the residue was dissolved in 300ml of ethanol, 5N hydrochloric acid was added, and heated to reflux for 1 hour. After the reaction solution was cooled, the solvent was removed under reduced pressure, the residue was neutralized with saturated sodium bicarbonate to pH=7-8, the aqueous phase was extracted w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com