Preparation method for cabazitaxel
A cabazitaxel and compound technology, applied in the field of preparation of cabazitaxel, can solve the problems of complex process, harsh reaction conditions, low yield and the like, and achieve the effects of simple process, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of Cabazitaxel
[0043] A preparation method of cabazitaxel, the route of synthesizing cabazitaxel is to use compound 1 (as shown in the reaction formula) as a raw material, the protective groups at positions 7 and 10 are removed, and then double methylation, and finally 13 The side chain is hydrolyzed to open the ring to obtain compound 4 Cabazitaxel.
[0044] Specifically include the following steps:
[0045] (1) Compound 1 is deprotected to release the hydroxyl groups at positions 7 and 10 to synthesize compound 2.
[0046] Take a 2.0L three-necked bottle, add 750mL of methanol and 120mL of acetic acid, heat the solution to 45°C, add compound 1 (100g) and zinc powder (60g) to the above solution, and react for about 5min. Filter, pour the filtrate into cooled water, stir, and a large amount of white solid is precipitated, which is filtered to obtain a white solid, which is dissolved in ethyl acetate with NaHCO 3 The solution was adjusted to ne...
Embodiment 2
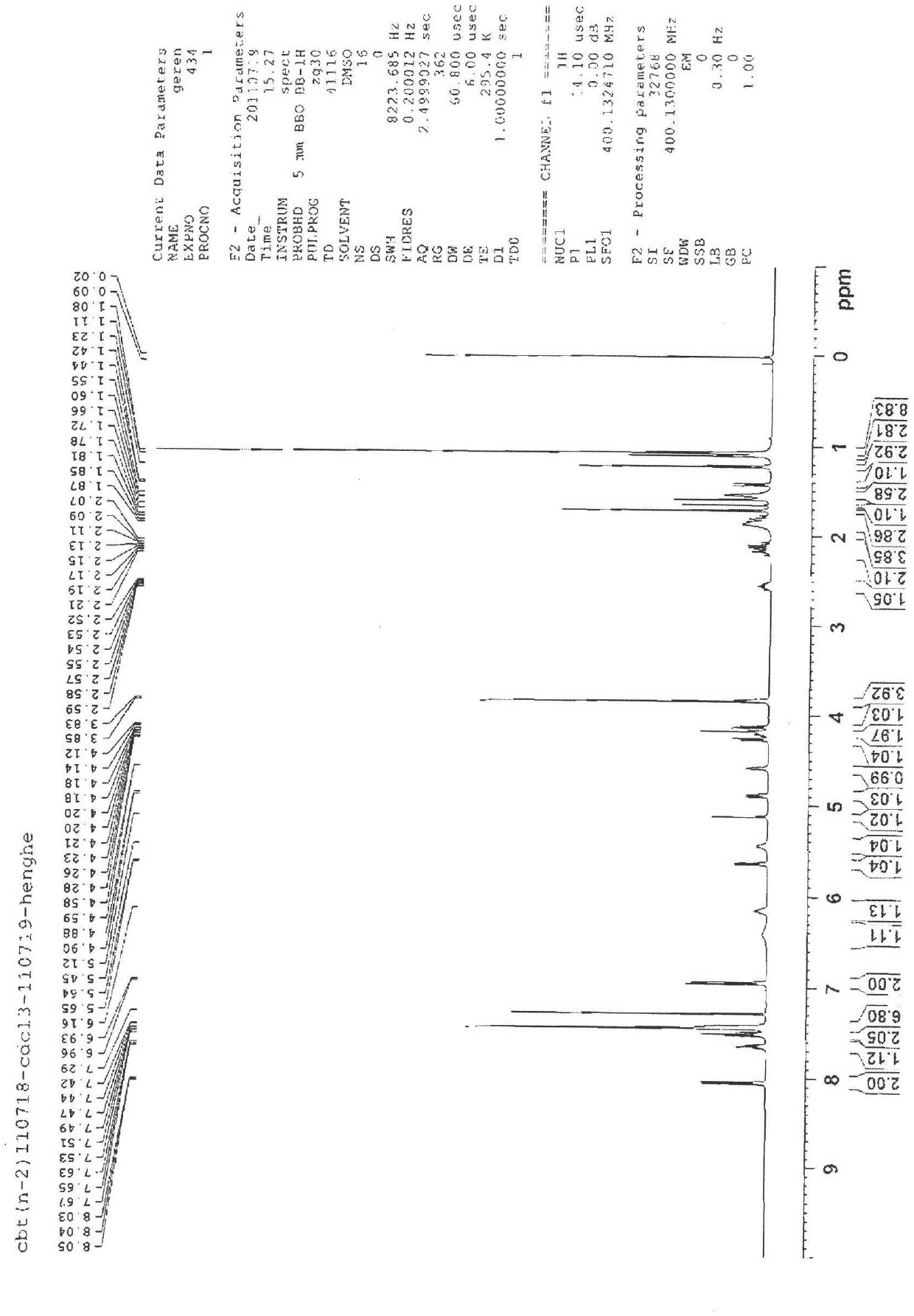
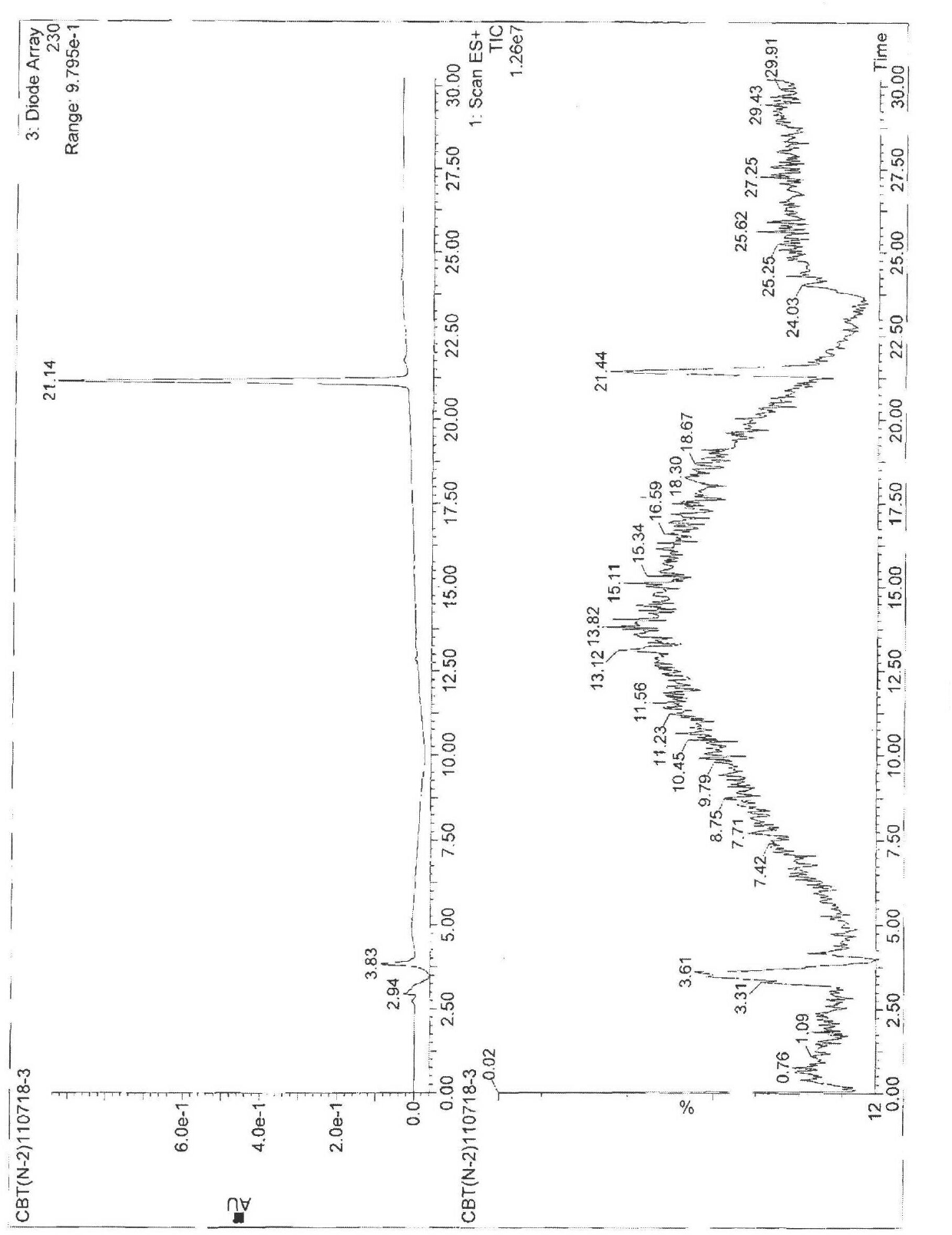
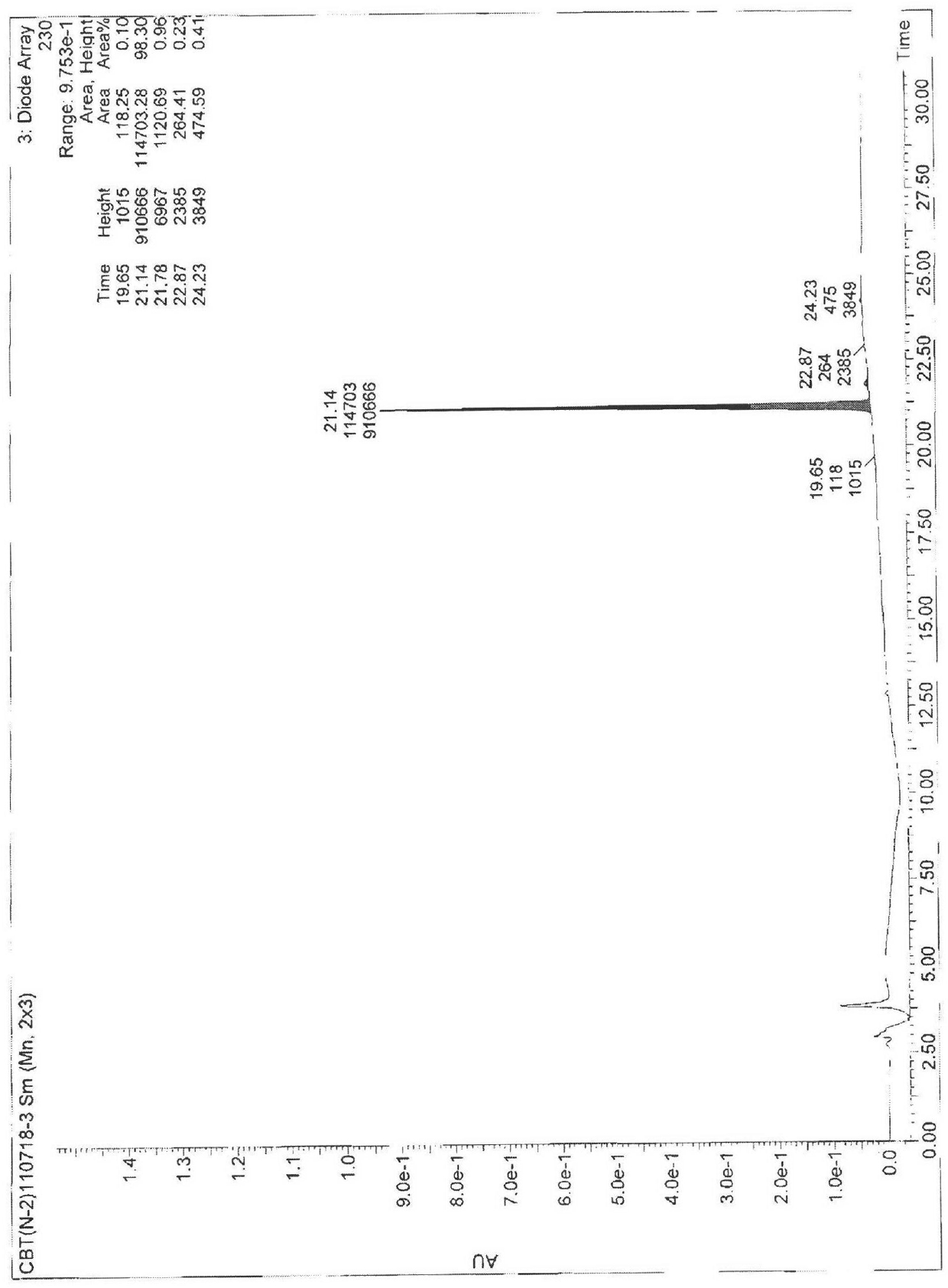
[0061] Example 2 Test for the purity of cabazitaxel
[0062] The purity of compound 4 was tested by HPLC as Figure 13 shown and shown in Table 1, Figure 13 HPLC for the purity test of compound 4.
[0063] Table 1 Parameters of the HPLC spectrum of the product purity prepared by the present invention
[0064]
[0065] In patent US5847170A, the synthetic method of cabazitaxel is reported, but the steps are cumbersome. It takes 10-DAB (III) as the initial raw material, and uses trimethylsilicon to protect the 7 and 13 hydroxyl groups first and then carry out the methylation of the 10 position. , then remove the 7 and 13 protecting groups, then methylate the 7-position hydroxyl, and finally couple with the side chain and open the ring to obtain the product cabazitaxel, and the total weight yield is less than 20% (with 10-DAB (III) ) is the raw material), because the steps are cumbersome, the used raw materials, solvents and various energy consumptions are extremely large, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com