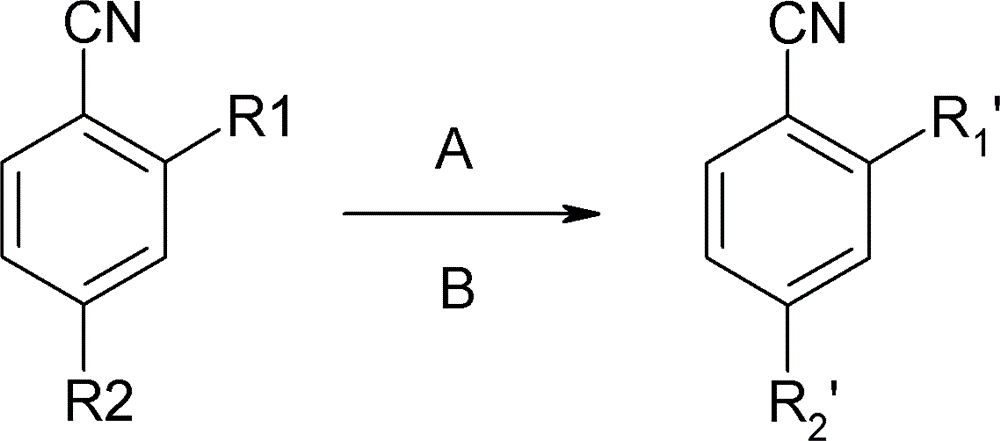
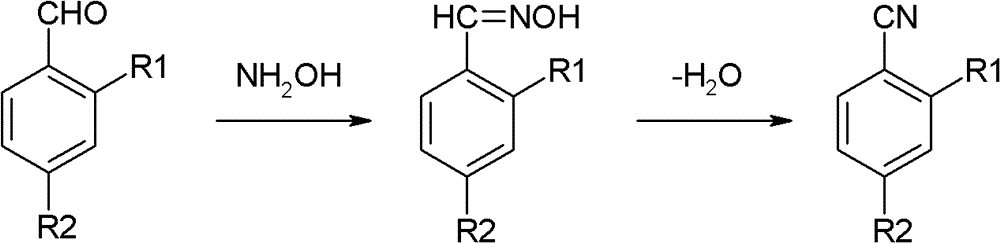
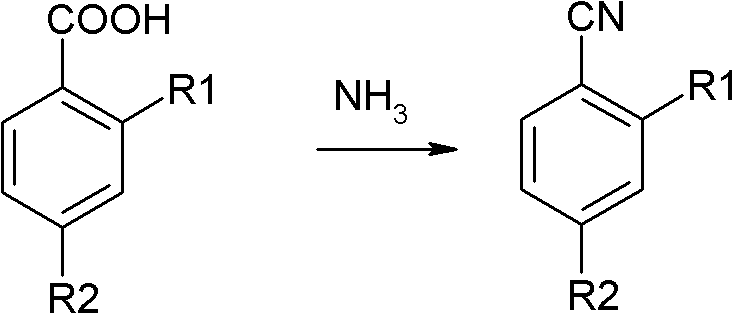
Preparation method of o(p)-hydroxybenzonitrile
A technology of hydroxybenzonitrile and halogenated benzonitrile is applied in the field of preparation of important fine chemical intermediate o-hydroxybenzonitrile, which can solve the problems of being unsuitable for large-scale production, difficult to obtain, etc. The effect of less product and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]Add 250g of sodium methoxide methanol solution with a solution concentration of 20.7% and 60g of o-chlorobenzonitrile into a 1000ml autoclave, replace the air in the autoclave with nitrogen, raise the temperature to 160°C, and the autoclave pressure to 2.5Mpa, and react in this state After 6 to 9 hours, the reaction is completed, the temperature is lowered to below 50°C, and methanol is recovered under reduced pressure (applicable), adding cold water to dissolve the residual product, filtering the insoluble matter, acidifying with 36% hydrochloric acid, and the white product o-hydroxybenzene is precipitated Amethonitrile, the content after drying is >99.0%, the mass is 50.5g, and the yield is 96.4%.
Embodiment 2
[0056] Add 258g of 28.5% sodium methoxide methanol solution and 75g of p-chlorobenzonitrile into a 1000ml autoclave. After replacing the air in the autoclave with nitrogen, the temperature is raised to 200°C, and the autoclave pressure is raised to 2.8Mpa. React in this state for 5 ~7 hours, finish the reaction, lower the temperature to below 50°C, recover methanol under reduced pressure (applicable), add cold water to dissolve the residual product, filter the insoluble matter, acidify with 36% hydrochloric acid, and precipitate the white product p-hydroxybenzonitrile, After drying, the content is >99.0%, and the mass is 62.8g. Yield 95.9%.
Embodiment 3
[0058] Add 244g of ethanol solution with a solution concentration of 25.7% sodium ethoxide and 70g of o-bromobenzonitrile in a 1000ml autoclave, replace the air in the autoclave with nitrogen, raise the temperature to 200°C, and the autoclave pressure to 3.0Mpa, and react in this state After 5-7 hours, finish the reaction, lower the temperature to below 50°C, recover ethanol under reduced pressure (applicable), add cold water to dissolve the residual product, filter the insoluble matter, acidify with 36% hydrochloric acid, and precipitate the white product o-hydroxybenzonitrile , the content after drying is >99.0%, and the mass is 43.5g. Yield 96.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com