Method for Synthesizing Chiral Alcohols by Asymmetric Reduction of Prochiral Carbonyl Compounds by Optical Drive Biocatalysis
A technology of carbonyl compounds and biocatalysis, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., to achieve the effect of simple cultivation conditions and low-cost production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
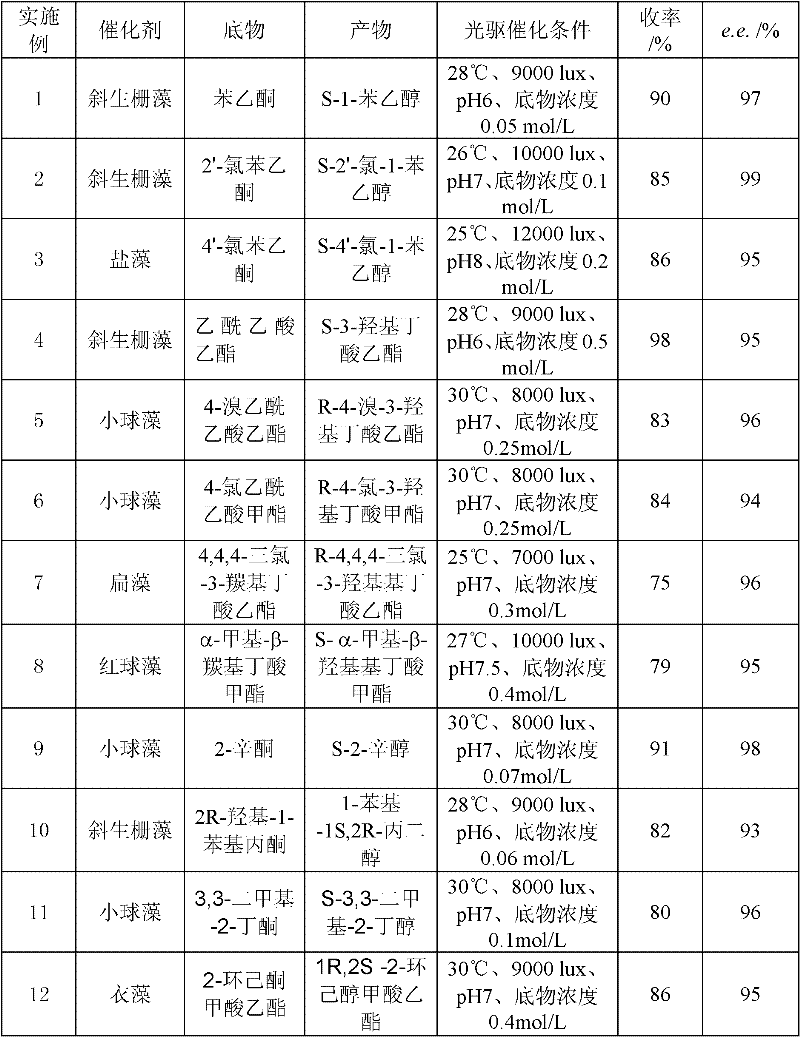
Examples
specific Embodiment approach
[0017] This embodiment comprises the following steps:
[0018] 1. Algae cultivation
[0019] Add 1 part of algae seed solution and 49 parts of culture medium into the photosynthetic reactor, and continuously pass CO into the photosynthetic reactor 2 The volume ratio to air is 5%-10%: 95%-90% of the mixed gas, the ventilation rate is 0.1vvm (vvm is the gas flow unit per minute per culture volume), the temperature is 25-30°C, and the light The intensity is 7000-12000lux, and the condition of pH6-8 is cultivated for 2-9 days.
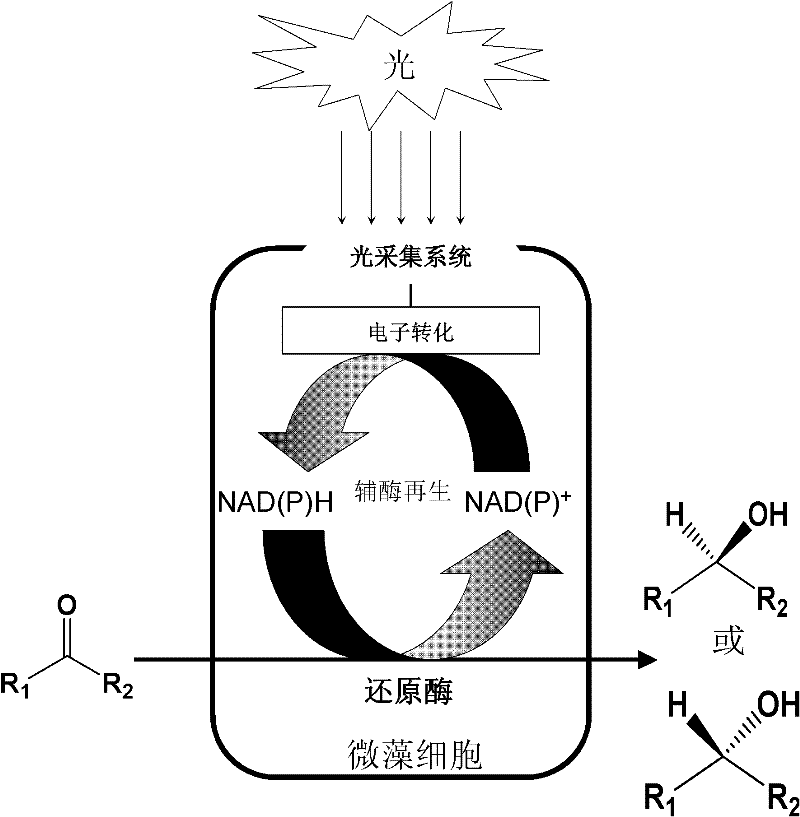
[0020] 2. Chlorella catalyzes the asymmetric reaction process of prochiral ketones
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com