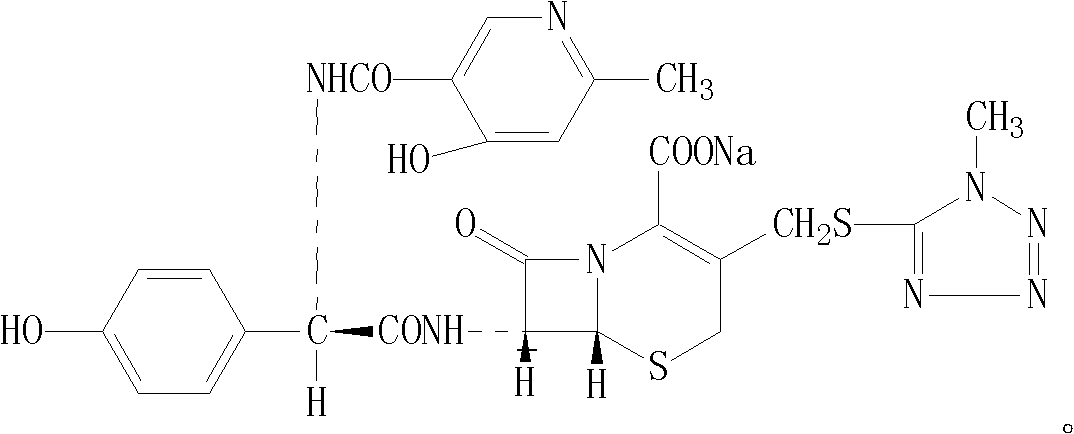
Cefpiramide sodium powder composition and preparation method thereof
A technology of cefpiramide and composition, applied in the field of cephalosporin drug powder injection composition, can solve the problems of high corrosion of equipment, slow dissolution speed, environmental pollution, etc., achieve fast dissolution speed, improved dissolution performance, and convenient clinical use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
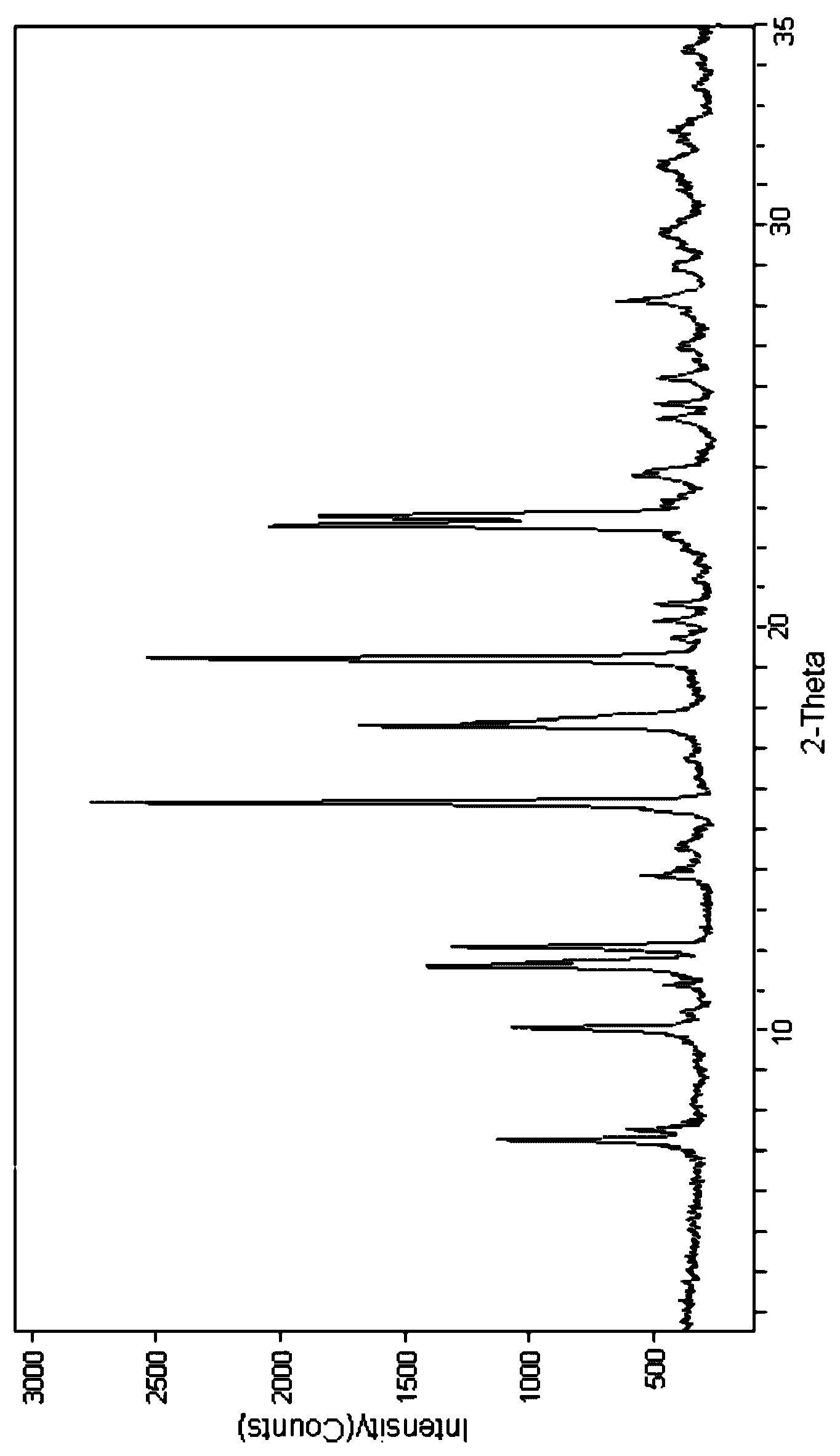
[0019] 2.5g cefpiramide is dissolved in the mixed solvent (the volume ratio of dimethylformamide and absolute ethanol is 10: 1) of the dimethylformamide of 5ml and dehydrated alcohol, first with speed be 1.5 ℃ / min Reduce the cefpiramide solution to 5°C at a speed of 0.5°C / min, then drop the cefpiramide solution to -5°C at a speed of 0.5°C / min, and stand at -5°C for 2 hours to obtain white crystals, filter, and filter cake After washing with acetone for 3 times and vacuum drying for 2 hours, cefpiramide crystals were obtained. After testing, the content of dimethylformamide in the obtained cefpiramide crystals was lower than 0.075%. Carry out X-ray powder diffraction to gained cefpiramide crystal (see figure 1 ), the diffraction peaks are displayed at the diffraction angles of 7.27°, 10.0°, 11.55°, 12.01°, 15.67°, 17.65°, 19.23°, 22.50°, 22.84°.
Embodiment 2
[0021] 2.5g cefpiramide is dissolved in the mixed solvent (the volume ratio of dimethylformamide and absolute ethanol is 12: 1) of the dimethylformamide of 5ml and dehydrated alcohol, first with speed be 1.0 ℃ / min Reduce the cefpiramide solution to 10°C at a speed of 0.7°C / min, then drop the cefpiramide solution to -10°C at a speed of 0.7°C / min, and stand at -10°C for 2 hours to obtain white crystals, filter, and filter cake After washing with acetone for 3 times and vacuum drying for 2 hours, cefpiramide crystals were obtained. After testing, the content of dimethylformamide in the obtained cefpiramide crystals was lower than 0.075%. The obtained cefpiramide crystals were analyzed by X-ray powder diffraction and hydrogen nuclear magnetic resonance, and the results were consistent with the obtained cefpiramide crystals in Example 1.
Embodiment 3
[0023] 2.5g cefpiramide is dissolved in the mixed solvent (the volume ratio of dimethylformamide and absolute ethanol is 15: 1) of the dimethylformamide of 5ml and dehydrated alcohol, first with speed be 1.2 ℃ / min Reduce the cefpiramide solution to 5°C at a speed of 0.5°C / min, then drop the cefpiramide solution to -7°C at a speed of 0.5°C / min, and stand at -7°C for 1 hour to obtain white crystals, filter, and filter cake After washing with acetone for 3 times and vacuum drying for 1 hour, cefpiramide crystals were obtained. After testing, the content of dimethylformamide in the obtained cefpiramide crystals was lower than 0.075%. The obtained cefpiramide crystals were analyzed by X-ray powder diffraction and hydrogen nuclear magnetic resonance, and the results were consistent with the obtained cefpiramide crystals in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com