Quinacridone derivatives and uses thereof
A technology for quinacridone and derivatives, which is applied in the field of quinacridone derivatives, and can solve problems such as expensive preparation process and restrictions on widespread use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
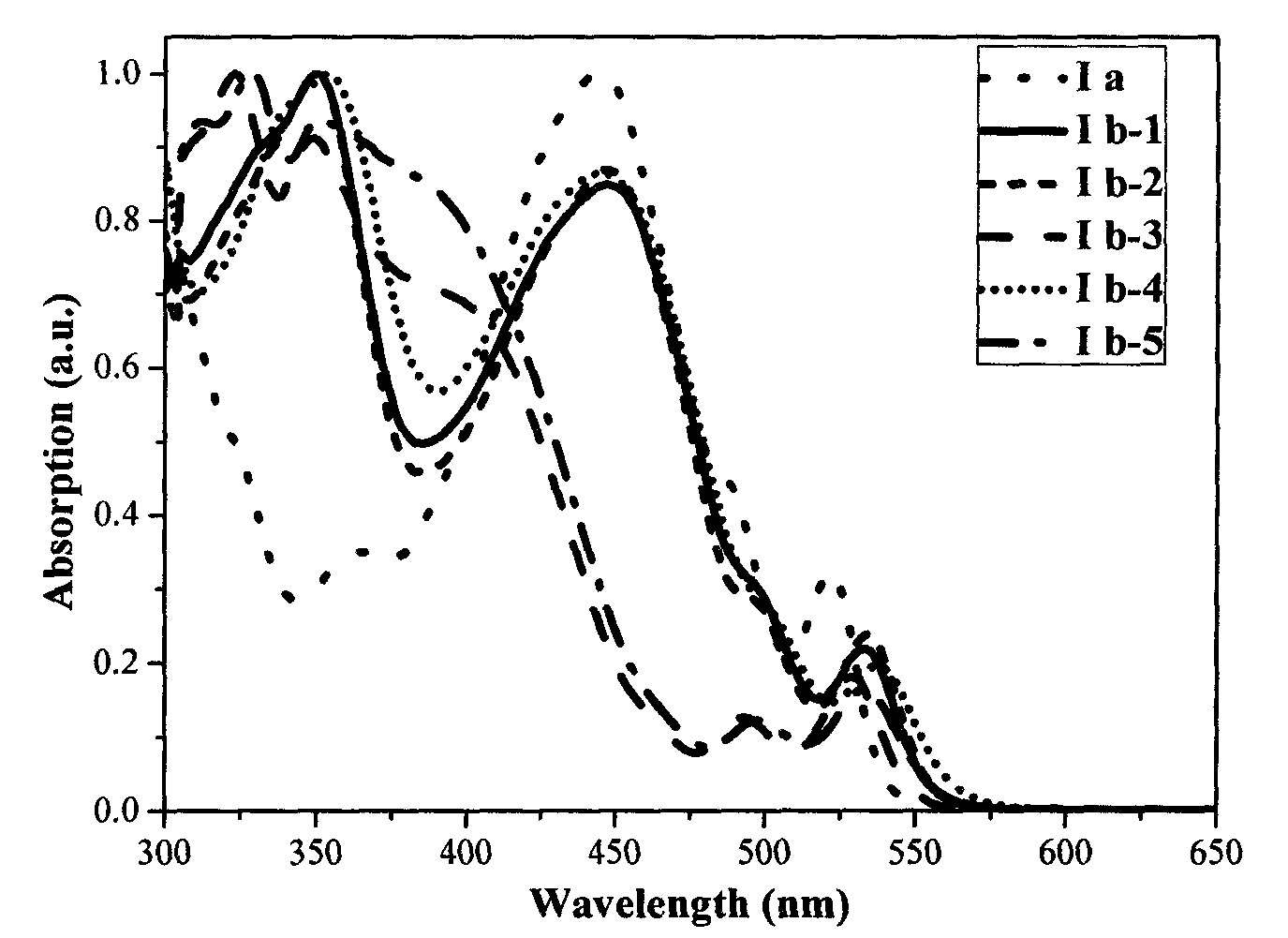
Image
Examples
Embodiment 1 and Embodiment 2
[0045]
[0046] With the teaching of the above preparation methods, those skilled in the art can prepare other compounds contained in formula I without creative effort.
[0047] Application of quinacridone derivatives provided by the invention in the preparation of photosensitizers for dye-sensitized solar cells:
[0048]The preparation of the battery is firstly to pretreat the conductive glass, and then apply the titanium dioxide slurry on the treated conductive glass, after high temperature treatment, immerse in the dye bath and absorb for 12 hours to form the working electrode of the battery. A dye-sensitized solar cell was prepared by encapsulating the configured electrolyte between the working electrode and the platinum-coated counter electrode with a sealant. The test of battery performance is to lead wires from the working electrode and counter electrode of the battery respectively, and connect them to the battery performance testing device. The working area of the...
Embodiment 1
[0053]
[0054] Add 4.68g quinacridone (15mmol) and 2.88g NaH (120mmol) into a 250mL three-neck flask, inject 100mLTHF under the protection of argon, and heat to reflux for 1 hour. Then inject 11.58g bromooctane (60mmol), reflux reaction for 24 hours. After the reaction was completed, the reaction solution was poured into water to precipitate a solid, which was filtered with suction, and the crude product was purified on a silica gel column (petroleum ether:dichloromethane=1:1) to obtain an orange solid i with a yield of 62%.
[0055] 1 H NMR (CDCl 3 , 400MHz), δ: 0.90(t, 6H), 1.30(m, 12H), 1.47(m, 4H), 1.61(m, 4H), 2.03(m, 4H), 4.51(t, 4H), 7.28( m, 2H), 7.51(m, 2H), 7.75(m, 2H), 8.58(dd, J 1 =1.9Hz,J 2 =2.2Hz, 2H), 8.77(d, J=3.7Hz, 2H).
[0056]
[0057] Add 1.08g compound i (2mmol), 356mgNBS (2mmol) and 50mLCCl in 100mL one-necked flask 4 , reflux reaction for 12 hours under the protection of argon. After the reaction was complete, the solvent was removed and a...
Embodiment 3
[0079]
[0080] Add 258mg XII-1 (0.3mmol) to a 250mL three-necked flask, a small amount of Pd(PPh 3 ) 4 , 12.5mL 2mol.L -1 K 2 CO 3 Solution and 25mL tetrahydrofuran, under the protection of argon, heated to reflux for half an hour, then poured into 25mL tetrahydrofuran solution containing furan boronic acid aldehyde (84mg, 0.6mmol), and reacted under reflux for 12 hours. After the reaction was completed, the liquid was separated and the water layer was removed, spin-dried, then extracted with water and dichloromethane, the oil phase was taken out, the solvent was removed and then applied to a silica gel column (developing solvent was dichloromethane) to obtain 250 mg of a red solid (compound XIII-2) , yield 95.4%.
[0081] 1 H NMR (CDCl 3 , 400MHz), δ: 0.90(m, J=6.7Hz, 6H), 1.36(m, 12H), 1.50(m, 4H), 1.64(m, 4H), 2.01(m, 4H), 4.51(m, 4H), 6.88(d, J=3.7Hz, 1H), 7.06(t, J=7.3Hz, 2H), 7.17(m, 6H), 7.29(m, 4H), 7.34(d, J=3.7Hz, 1H), 7.52(m, 4H), 7.88(d, J=9.1Hz, 1H), 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com