Crystal form of lenalidomide and preparation method thereof
A technology of lenalidomide and crystal form, applied in antipyretic drugs, antineoplastic drugs, organic chemistry, etc., can solve the problems of increasing the complexity of the manufacturing process and increasing the manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1 : Preparation of anhydrous lenalidomide according to the first aspect of the invention from 1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-4-nitro-isoindole amine.
[0107] Suspend 1,3-dioxo-2-(2,6-dioxopiperidin-3-yl)-4-nitro-isoindole (10.0 g, 0.035 mol) in ethanol (120 ml), And iron powder (9.6 g, 0.175 mol) was added thereto. Hydrochloric acid (18.5 ml) diluted with an equal volume of water was added to the mixture. The reaction mixture was heated to about 65-70 °C and maintained at this temperature for about 1 1 / 2 -2 hours. Cool the reaction mixture and pass Filter through a pad and concentrate the resulting clear filtrate. The pH of the filtrate was adjusted to 7 to 8 with ammonia solution and passed The concentrated filtrate was further filtered through a pad. The filtrate was concentrated again under reduced pressure. The product was finally dissolved in ethanol (50ml) and heated at 45-50°C until a clear solution formed. The solution was cooled ...
Embodiment 2
[0111] Example 2 : The anhydrous crystalline form of lenalidomide according to the first aspect of the present invention is converted into the crystalline form B of lenalidomide of the prior art.
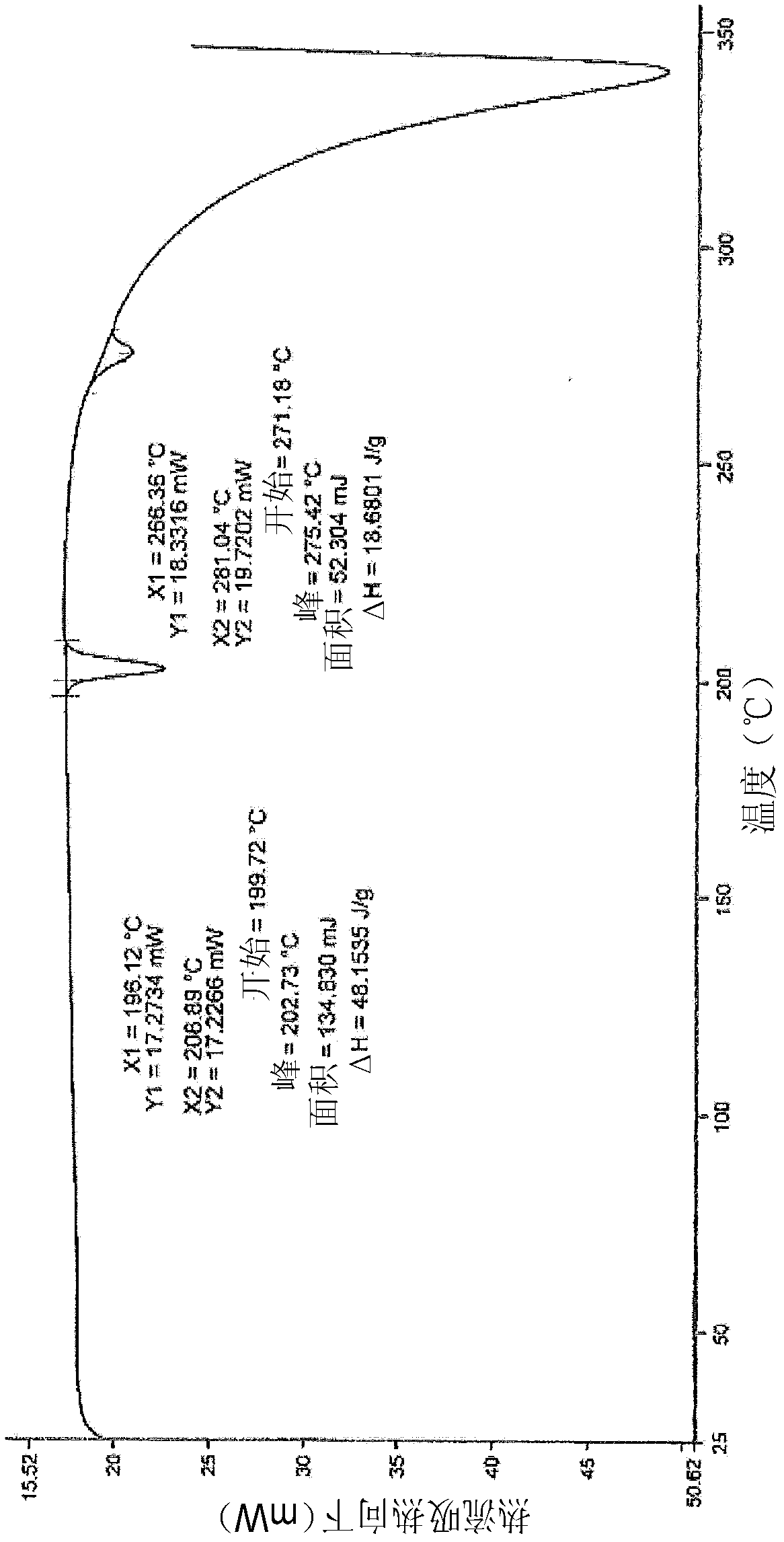
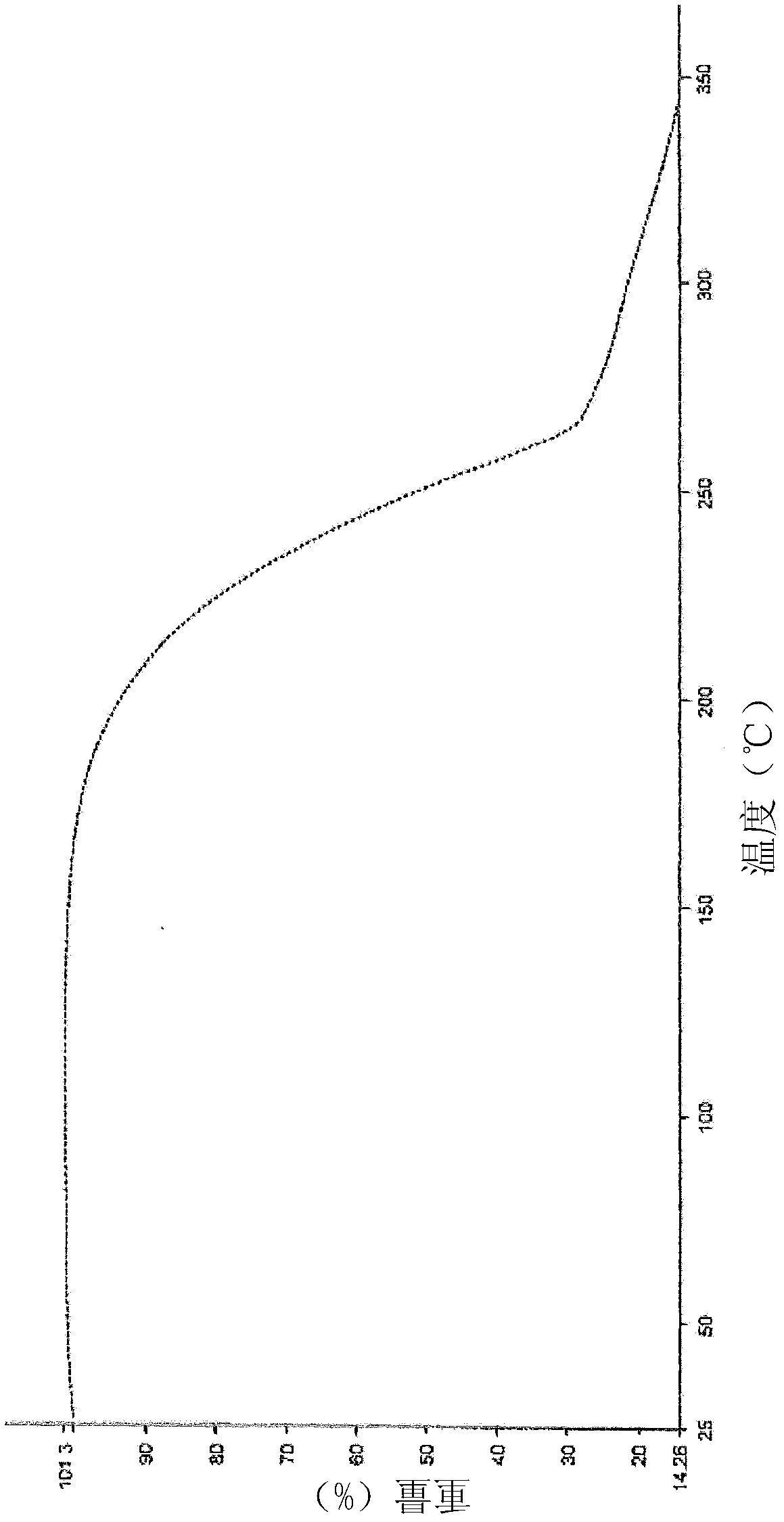
[0112]Anhydrous crystalline lenalidomide (1.5 g) prepared according to Example 1 was dissolved in a mixture of methanol:water (80:20) (22.5 ml) by heating the reaction mixture at 45-50° C. until a clear solution was formed . The solution was cooled slowly to about 25-30°C. When the solution started to appear cloudy, it was cooled to about 0-5°C and held at temperature while stirring at this temperature for 2-3 hours. The crystalline product was filtered, washed with cold methanol (3ml), then dried by vacuum filtration. Finally the product was dried at 45-50°C for about 3 hours under a pressure of 100 mmHg. The resulting dried solid was subjected to XRPD, DSC and TGA analysis, which confirmed that Form B had been prepared as disclosed in WO 2005 / 023192.
[0113] The chemical pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com