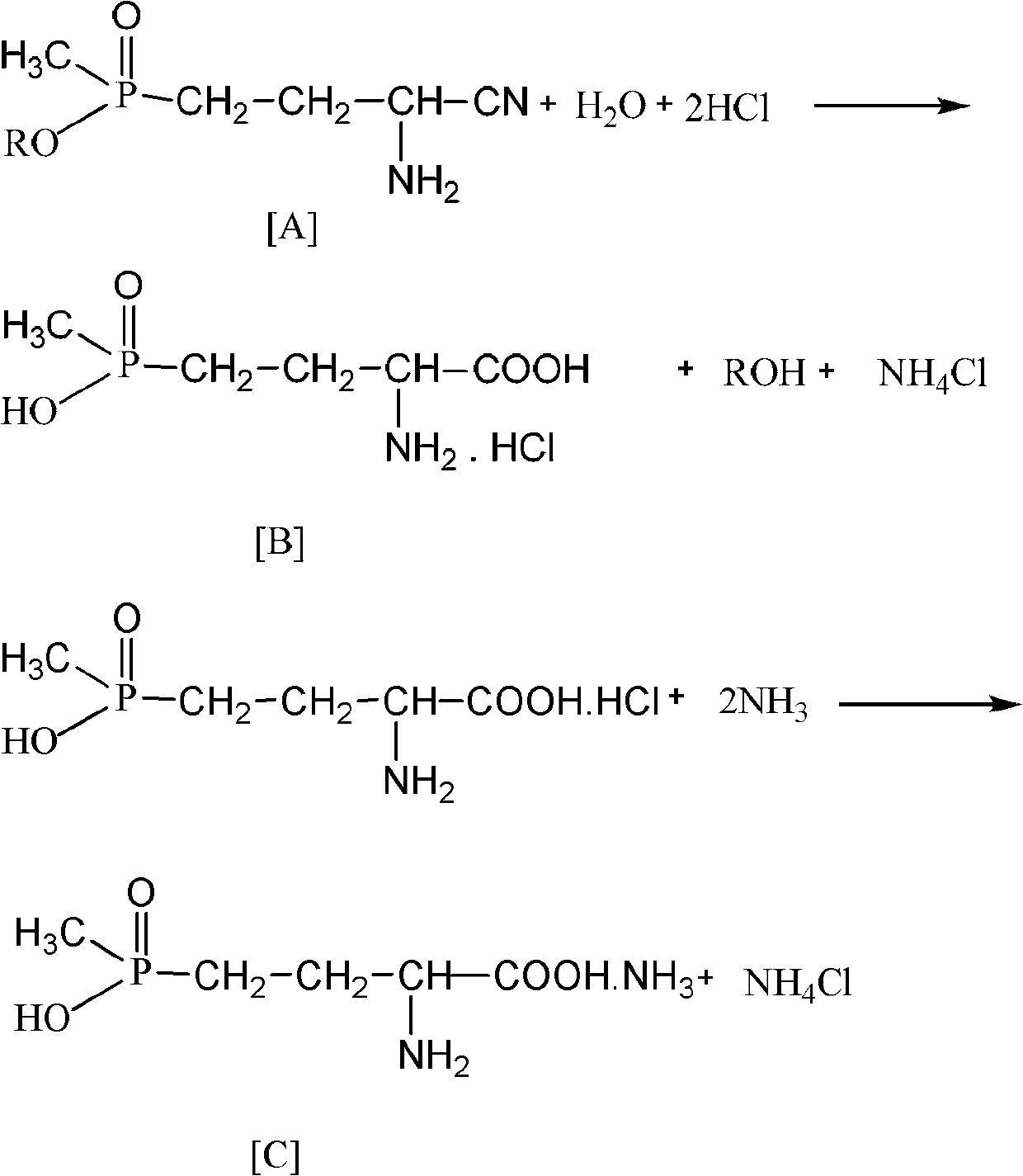
Process for purifying glufosinate-ammonium
A technology for glufosinate-ammonium and glufosinate-ammonium hydrochloride, which is applied in the fields of compounds of Group 5/15 elements of the periodic table, organic chemistry, chemical instruments and methods, etc., can solve the problem that it is difficult to prepare and separate high-purity glufosinate phosphine and other problems, to achieve the effect of low inorganic salt content, simple process steps and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
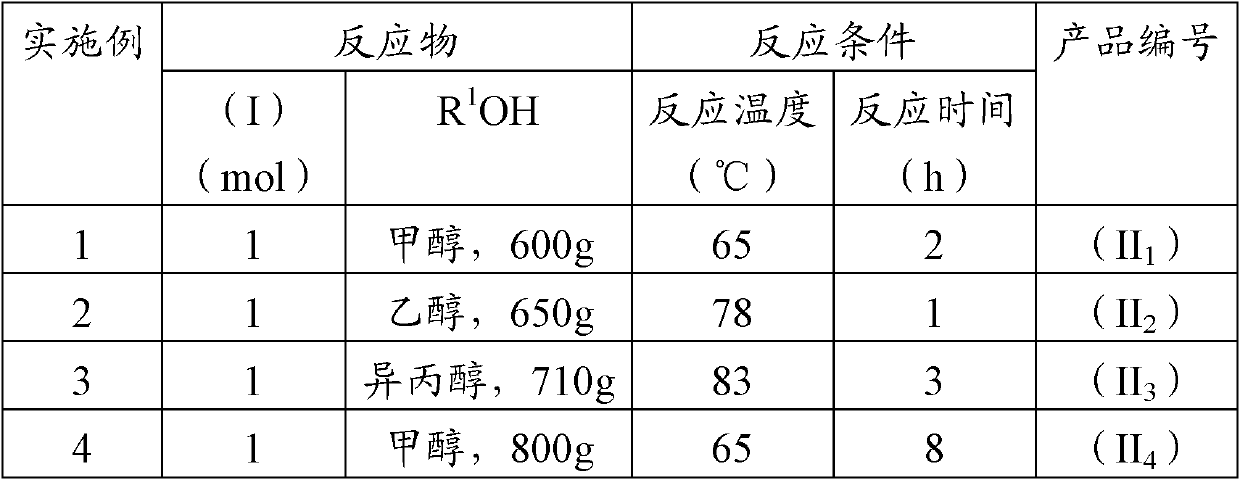
[0021] Add glufosinate-ammonium hydrochloride (I) to alcohol R according to the feeding ratio in Table 1. 1 OH, carry out the esterification reaction, after the reaction is complete, cool to below 15°C, filter to remove insoluble matter in the system (the white solid is NH 4 Cl and a small amount of sodium chloride), the filtrate is decompressed to remove the solvent to obtain the esterified product (II) of glufosinate-ammonium hydrochloride, and the evaporated alcohol solvent can be recycled and reused;
[0022] Table 1 Feeding ratio and reaction conditions of esterification reaction
[0023]
[0024] According to the ratio and reaction conditions in Table 2, the esterified product of glufosinate-ammonium hydrochloride prepared in Examples 1-4 (II 1 )-(II 4 ) Were added to the hydrochloric acid aqueous solution to carry out the hydrolysis reaction. After the reaction was completed, the acid water was removed, 350g of ethanol was added and stirred at 50°C for 3 hours to gradually dis...
Embodiment 5-8
[0028] According to the feed ratio in Table 3, the glufosinate-ammonium hydrochloride (III) finally obtained in Examples 1 to 4 was added to the alcohol R 2 Then pass ethylene oxide into OH, after the reaction is complete, cool to 0~5°C and filter to obtain wet white crystalline glufosinate ammonium phosphonic acid, which is dried to obtain glufosinate ammonium phosphonic acid (IV);
[0029] table 3
[0030]
[0031]
Embodiment 9-12
[0033] According to the feeding ratio in Table 4, the glufosinate (IV) obtained in Examples 5 to 8 was added to R 3 In OH, pass ammonia gas at 30-40°C, complete the reaction, cool to 0°C and filter to obtain wet product, and dry to obtain glufosinate-ammonium;
[0034] Table 4
[0035]
[0036] Among them, the final structure detection data of glufosinate-ammonium is:
[0037] 1 H-NMR (400MHz, D 2 O, TMS): δ=3.785 (1H, t), 2.065 (2H, t), 1.542-1.727 (2H, m), 1.253 (3H, d);
[0038] 13 C-NMR(400MHz, D 2 O, TMS): δ=176.51(s), 57.57(d), 29.41(d), 26.56(s), 17.32(d);
[0039] 31 P-NMR(400MHz, D 2 O, TMS): δ=43.846(s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com