Serine protease inhibitor pp-pi polypeptide from chrysalis chrysalis chrysalis venom and its application
A technology of serine protease, golden bee, applied in the direction of protease inhibitor, application, peptide source, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] 1. Isolation and N-terminal sequencing of chrysalis chrysalis venom protein components:
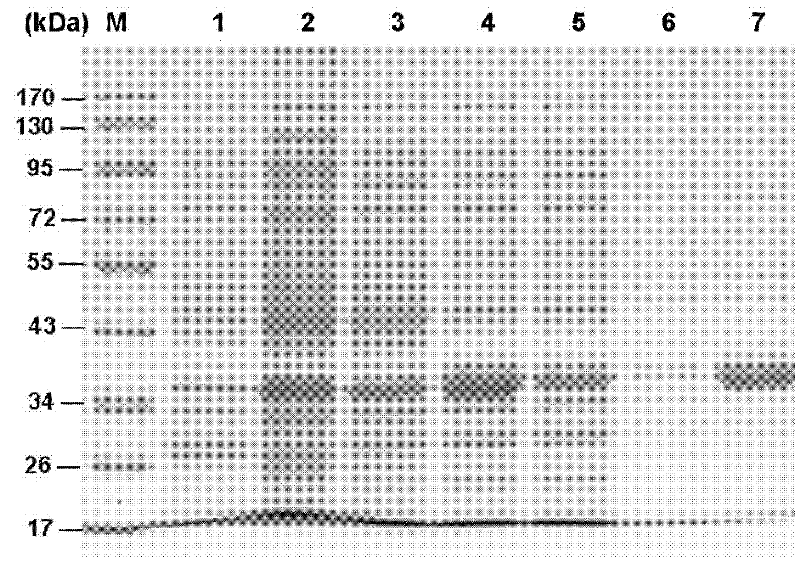
[0076] The female Pteromalus puparum (Pteromalus puparum) was dissected in PBS (including PMSF) on ice with an Olympus dissecting microscope and a dissecting needle. The venom sac and venom gland were centrifuged at 16000g for 1 min to obtain the supernatant, which was freeze-dried and dissolved in deionized water as a sample for the separation of venom proteins.
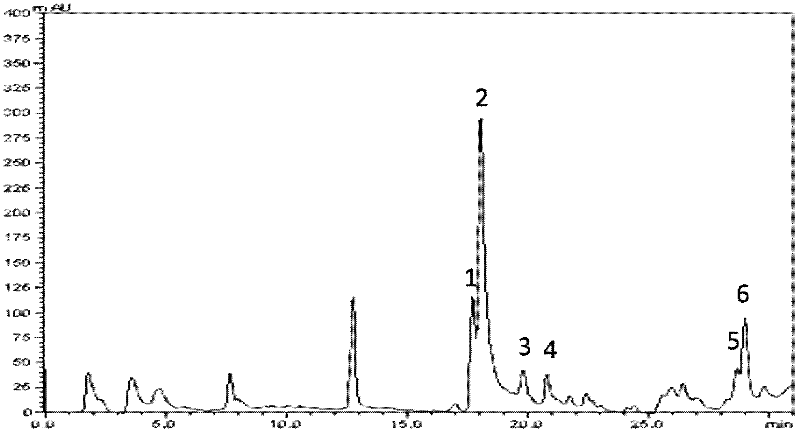
[0077] The chromatographic column is an Aligent C18 column (15cm×4.6mm, particle size 5 μm); the mobile phase is trifluoroacetic acid (TFA) (A--1: 1000, namely containing TFA0.1% aqueous solution) and acetonitrile (B), in 40min The internal acetonitrile concentration ranges from 0 to 40% (volume concentration of acetonitrile), the flow rate is 1.0 mL / min, the detection wavelength is 214 nm, and single peaks are collected.
[0078] Entrust the Institute of Biophysics, Chinese Academy of Sciences to complete mass spectrom...
Embodiment 2
[0119] 1. Construction of PpPI gene plant binary expression vector
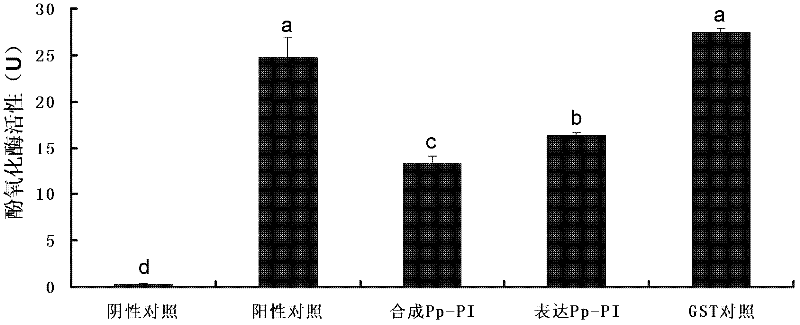
[0120] Using cauliflower mosaic virus CaMV 35S promoter and NOS terminator on both sides of GUS gene in plant binary expression vector pBI121, insert PpPI gene to form a complete expression framework. In this experiment, BamHI and SacI were selected as restriction sites to replace the GUS gene with PpPI, so that the expression boxes on both sides of the GUS gene were used to control the expression of the PpPI gene in Arabidopsis plants.
[0121] Primers with BamH I and Sac I restriction sites were designed according to the ORF of the serine protease inhibitor of the venom of A. chrysalis, and the plant expression vector was constructed by PCR amplification using the venom cDNA of A. chrysalis as a template. The primer sequences are:
[0122] PpPI-SP: TCG GGATCC ATTCTGTACAAGATGAGGACGAT,
[0123] PpPI-AP: CGC CTAAGAGCAATTCATTCGTGTG,
[0124] in, GGATCC is the BamH I restriction site, Sac I restrictio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com