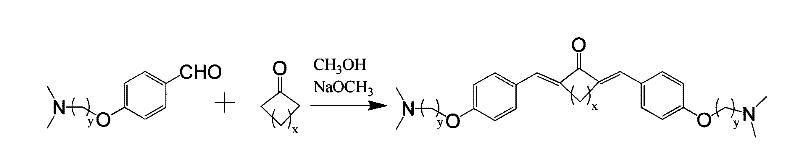Application of 2(ammonia alkyloxy) phenyl pentadiene ketone compound in preparation of drugs for treating chemical ache and/or immunologic injury
A technology of phenylpentadienone and aminoalkoxy, which is applied in the field of medicine and can solve problems such as easy dependence and reduced curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Synthesis of two (aminoalkoxy) phenyl pentadienone compounds
[0044] Synthetic general method: Dissolve 10 mmol of substituted benzaldehyde in 10 mL of absolute ethanol, stir at room temperature for 5 min, then add the corresponding ketone, continue stirring for 10 min, the solution remains unchanged. Sodium metal was dissolved in methanol to prepare 18% (w / v) sodium methoxide / methanol solution. Slowly add 1.5 mL of the sodium methoxide solution (containing 5 mmol of sodium methoxide) into the reaction solution. After stirring for 2 hours, a large amount of insoluble yellow matter appears. The reaction solution is detected by TLC, and the purple color of substituted benzaldehyde no longer appears under a 320nm ultraviolet lamp. Spots, the product spots are clear and yellow. Stop the reaction, filter the reaction solution, wash the product with water first, then wash twice with ice ethanol and ice acetone, and dry in vacuum at 30°C overnight to obtain a yello...
Embodiment 2
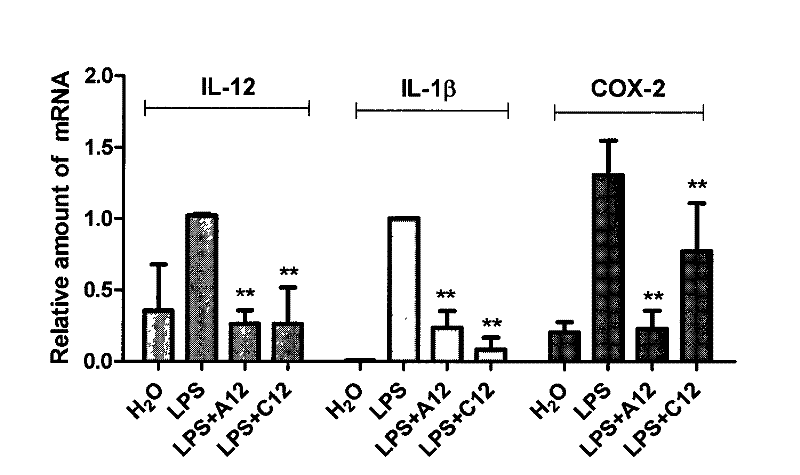
[0047] Example 2 Compounds A12 and C12 inhibit the production of IL-12, IL-1β and COX-2 (cyclooxygenase-2) mRNA in primary macrophages induced by LPS (lipopolysaccharide)
[0048] 1.2×10 6 Primary mouse macrophages were cultured at 37°C with 1640 culture medium. After 24 hours, the culture medium was renewed and different concentrations of compounds were added for pretreatment for 2 hours, and then treated with 0.5 μg / ml LPS for 6 hours, and the cells were collected and extracted. For total RNA, after reverse transcription, fluorescent quantitative PCR was used to detect the contents of IL-12, IL-1β and COX-2 mRNA, and β-actin mRNA was used as an internal reference to quantify genes. The result is as figure 2 As shown, compounds A12 and C12 significantly inhibited the increase of IL-12, IL-1β and COX-2 mRNA induced by LPS (there were significant differences, *p<0.05, **p<0.01).
Embodiment 3
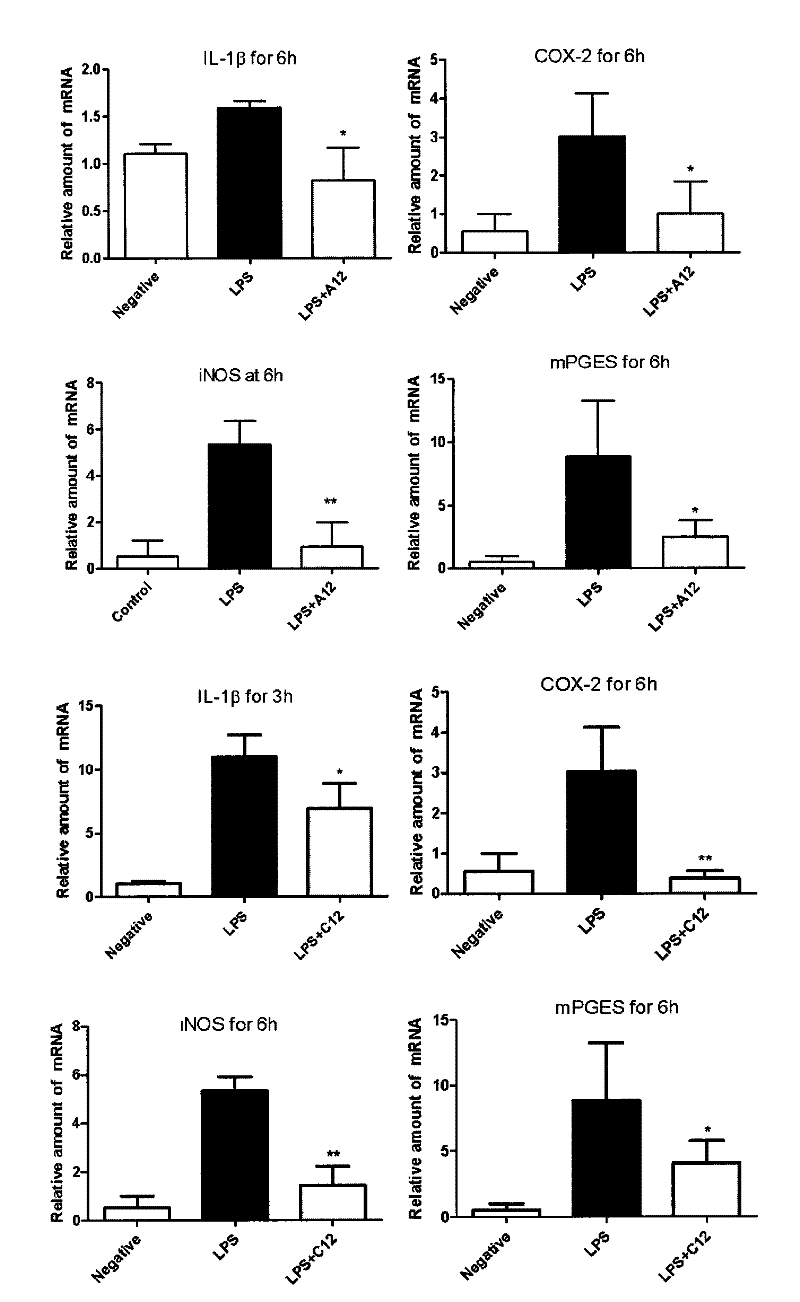
[0049] Example 3 Injection of compounds A12 and C12 inhibits the mRNA expression of IL-1β, iNOS (inducible nitric oxide synthase), COX-2 and mPGES (membrane-associated prostaglandin synthase) in LPS-induced mouse liver tissue
[0050] In order to further confirm that compounds A12 and C12 can inhibit the expression of various cytokines and enzymes induced by LPS in vivo, we measured IL-1β, iNOS, COX-2 and mPGES in mouse liver tissue at the mRNA level by RT-qPCR. mRNA levels. C57BL / 6 mice were first intravenously injected with A12 or C12 (15 mg / kg), and the control group was injected with the same dose of normal saline, and 15 minutes later, LPS (10 mg / kg) was injected intravenously. After different time, the mice were killed, the liver tissue was homogenized, and the total RNA was extracted, and the content of these related mRNAs, such as iNOS, COX-2, IL-1β and mPGES, were detected by RT-qPCR technology, and Actin was used as the control Gene. The result is as image 3 show...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com