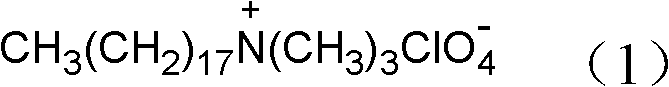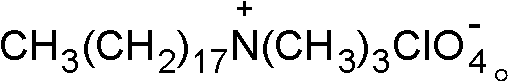Organic oxidizing agent and preparation method thereof
An organic oxidant and catalyst technology, applied in the field of pyrotechnics, can solve the problems of high decomposition temperature, difficult to ignite, high cost, and environmental pollution, and achieve the effect of solving the problem of easy extinguishment, low hygroscopicity, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] With stearyl tertiary amine, monochloromethane, sodium perchlorate as raw materials, isopropanol and distilled water as solvent, potassium hydroxide as catalyst, the specific steps are:
[0020] 1) Add 0.01mol of octadecyl tertiary amine to a reactor containing 125ml of isopropanol and 125ml of distilled water as a mixed solvent, then add 0.1mmol of potassium hydroxide, replace the air in the reactor with nitrogen, and then heat up to 45°C. Then pass through 0.01mol monochloromethane, and react at 0.4MPa for 4h;
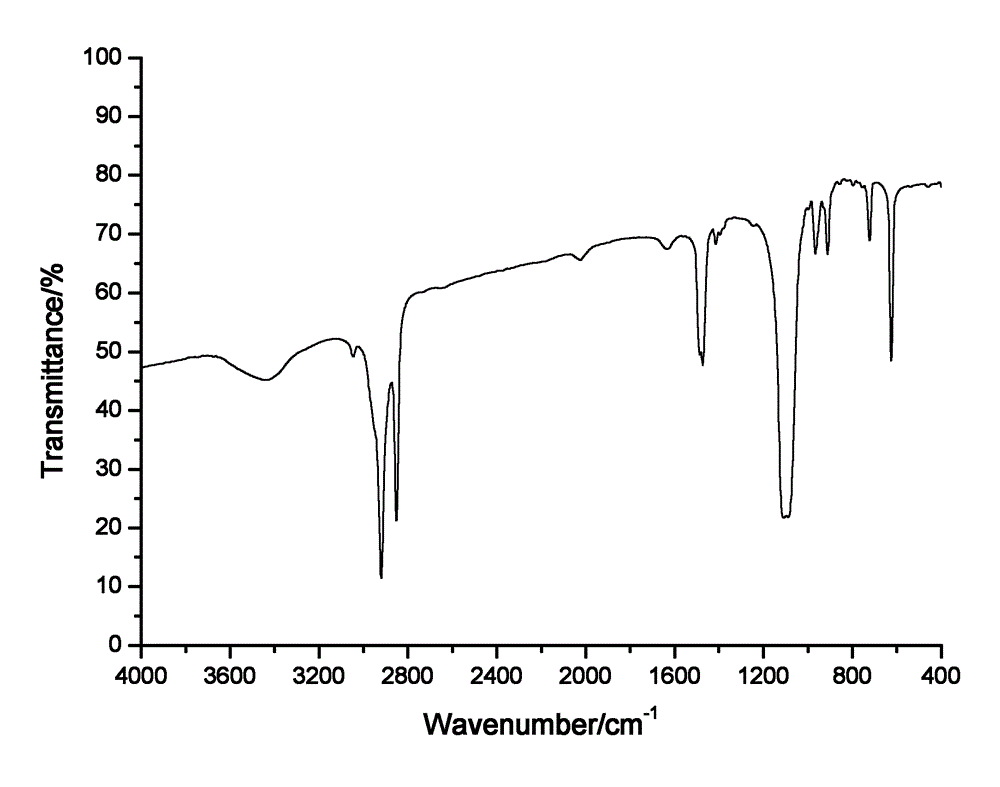
[0021] 2) Add 0.01 mol of sodium perchlorate into the reactor of step 1), and stir. After 10 hours of reaction, crystals are precipitated after cooling, filtered, and the filter cake is washed 3 times with distilled water, and dried in a vacuum oven at 60°C , to obtain the organic oxidant octadecyltrimethylammonium perchlorate, its molecular structure formula is Its FTIR spectrum is as figure 1 shown.
Embodiment 2
[0023] With stearyl tertiary amine, monochloromethane, sodium perchlorate as raw materials, isopropanol and distilled water as solvent, sodium hydroxide as catalyst, the specific steps are:
[0024] 1) Add 0.01mol of octadecyl tertiary amine to a reactor containing 83ml of isopropanol and 167ml of distilled water mixed solvent, then add 0.3mmol of sodium hydroxide, replace the air in the reactor with nitrogen, and then heat up to 45°C. Then pass through 0.01mol monochloromethane, and react at 0.4MPa for 4h;
[0025] 2) Add 0.01 mol of sodium perchlorate into the reactor of step 1), and stir. After 10 hours of reaction, crystals are precipitated after cooling, filtered, and the filter cake is washed 3 times with distilled water, and dried in a vacuum oven at 60°C , to obtain the organic oxidant octadecyltrimethylammonium perchlorate, its molecular structure formula is
[0026]
Embodiment 3
[0028] With stearyl tertiary amine, monochloromethane, sodium perchlorate as raw materials, isopropanol and distilled water as solvent, potassium hydroxide as catalyst, the specific steps are:
[0029] 1) Add 0.01mol of octadecyl tertiary amine to a reactor containing 62.5ml of isopropanol and 187.5ml of distilled water mixed solvent, then add 0.3mmol of potassium hydroxide, replace the air in the reactor with nitrogen and heat up to 55 ℃, then pass through 0.02mol monochloromethane, and react at 0.5MPa for 3h;
[0030] 2) Add 0.02 mol of sodium perchlorate into the reactor of step 1), and stir. After reacting for 15 hours, crystals precipitate out after cooling, filter, wash the filter cake 3 times with distilled water, and dry in a vacuum oven at 60°C , to obtain the organic oxidant octadecyltrimethylammonium perchlorate, its molecular structure formula is
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com