Formula of medicament for treating non-infectious ocular inflammations, and inhibiting corneal neovascularization and anti-rejection reaction generated after corneal transplantation
A corneal neovascularization, non-infectious technology, applied in the direction of medical formula, non-active ingredient medical preparations, medical preparations containing active ingredients, etc., can solve the problems of reduced dosage, high external environmental requirements, easy inactivation, etc. , to achieve the effect of improving activity, easy storage and good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
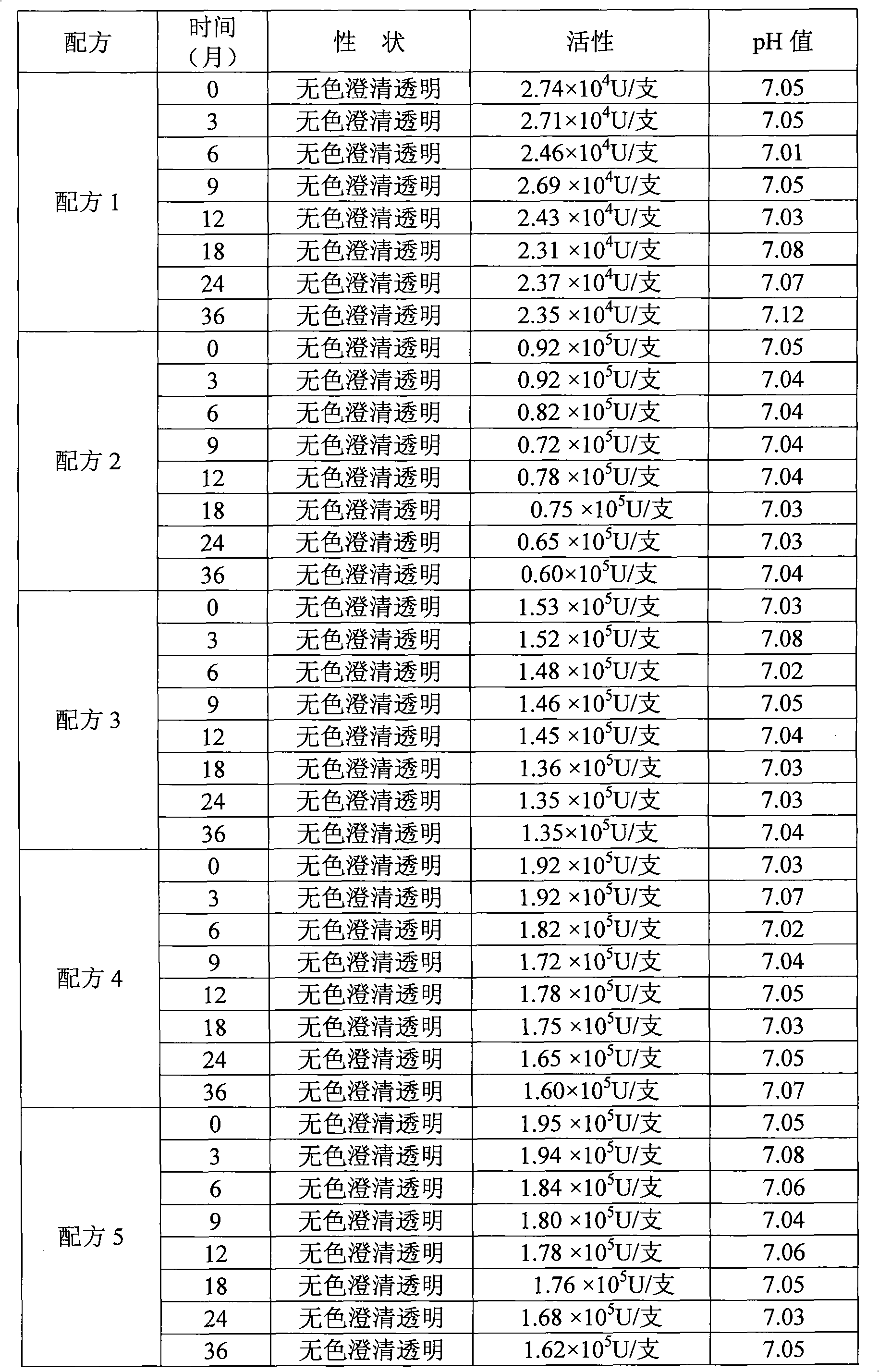
[0030] Embodiment 1 chooses that isotonic agent is glycerin and isotonic agent is the test result comparison of mannitol
[0031] Formula 1 (the existing formula used): 40 g of disodium hydrogen phosphate (buffer), 20 g of potassium dihydrogen phosphate (buffer), 30 g of potassium chloride (buffer), 70 g of mannitol, 2 g of human serum albumin, Recombinant human interleukin-1 receptor antagonist 50mg is prepared into 1000ml solution with sterile water.
[0032] Formula 2 Glycerin replaces mannitol, and the weight of glycerin is 10g.
[0033] Formula 3 Glycerin replaces mannitol, and the weight of glycerin is 20g.
[0034] Formula 4 Glycerin replaces mannitol, and the weight of glycerin is 30g.
[0035] Formula 5 glycerin replaces mannitol, and the weight of glycerin is 40g.
[0036] Choose every kind of formula solution to pack into 50 bottles, each bottle is 10 milliliters, and the test results are averaged.
[0037]
[0038] As can be seen from the results in Table 1,...
Embodiment 2
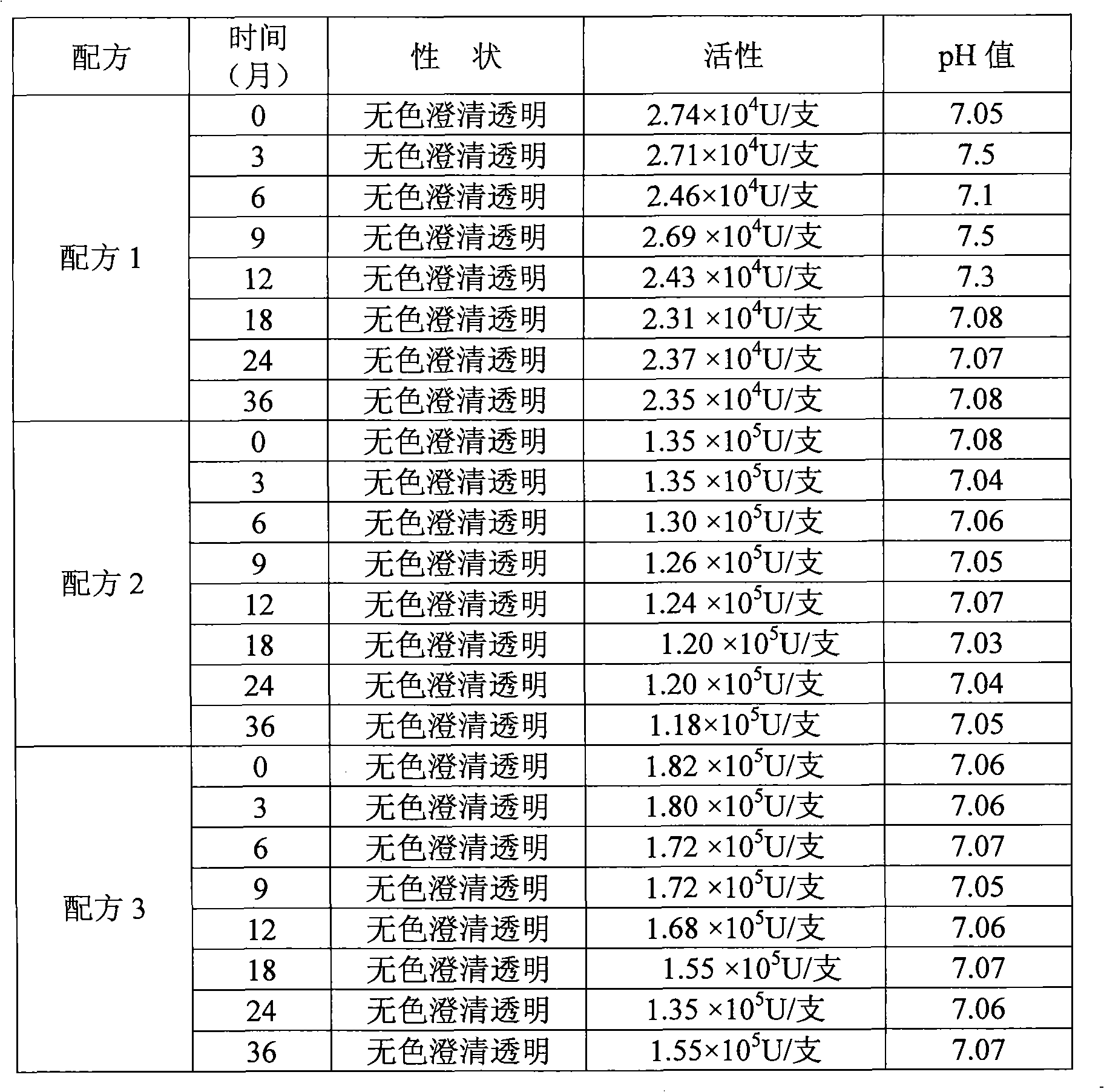
[0039] Embodiment 2 adopts the test result of sodium citrate and citric acid combination and disodium hydrogen phosphate and potassium dihydrogen phosphate contrast
[0040] Table 2 Comparison of different test results of buffer
[0041]
[0042] Formula 1 (the existing formula used): 40 g of disodium hydrogen phosphate (buffer), 20 g of potassium dihydrogen phosphate (buffer), 30 g of potassium chloride (buffer), 70 g of mannitol, 2 g of human serum albumin, Recombinant human interleukin-1 receptor antagonist 50mg is prepared into 1000ml solution with sterile water.
[0043] Formula 2 uses a combination of sodium citrate and citric acid as a buffer to replace the combination of disodium hydrogen phosphate and potassium dihydrogen phosphate, and the sodium citrate and citric acid are 40g and 20g respectively.
[0044] Formula 3 uses a combination of sodium citrate and citric acid as a buffer to replace the combination of disodium hydrogen phosphate and potassium dihydrogen...
Embodiment 3
[0047] Embodiment 3 adds preservative comparative test
[0048] Table 3 Adding preservatives and comparing the experimental results without adding
[0049]
[0050] Formula 1 (the existing formula used): 40 g of disodium hydrogen phosphate (buffer), 20 g of potassium dihydrogen phosphate (buffer), 30 g of potassium chloride (buffer), 70 g of mannitol, 2 g of human serum albumin, Recombinant human interleukin-1 receptor antagonist 50mg is prepared into 1000ml solution with sterile water.
[0051] Formula 2 adds 0.2 g of ethyl p-hydroxybenzoate on the basis of formula 1.
[0052] Choose every kind of formula, and solution is packed into 50 bottles, and every bottle is 50 milliliters, and test result is averaged.
[0053] From the test results in Table 3, it can be known that adding a preservative can better keep the recombinant human interleukin-1 receptor antagonist in a sterile and qualified state, and keep its activity stable for a longer period of time.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com