Preparation formula of gene therapy medicament taking recombinant adeno-associated virus (rAAV) as vector
A technology of gene therapy and preparation, applied in the field of molecular medicine and disease prevention and molecular pharmacology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of stock solution of rAAV gene medicine
[0021] To facilitate the determination of the transduction titer (biological activity) of the rAAV gene drug, the transgene of the drug in this example is the firefly luciferase (Fluc) gene (purchased from Promega).
[0022] The preparation method of the rAAV gene therapy vector used in this example is: preparation by multi-plasmid co-transfection method without the participation of helper virus, that is, the calcium phosphate method is used to combine the vector plasmid with the helper plasmid (such as H22, H23, H24, H25, H26, H27, H28, H29, pXX6, etc.) (purchased from the University of Pennsylvania) were co-transfected into 293 cells (purchased from the American Type Culture Collection (ATCC)), and the recombinant virus was harvested after 60-72 hours, and chlorinated cesium gradient centrifugation and purification for later use. AAV helper plasmids (H22, H23, H24, H25, H26, H27, H28, H29) and adenovirus helper pl...
Embodiment 2
[0028] rAAV gene drug transduction titer (biological activity) determination
[0029] ①Cultivate 293 cells in a 6-well cell culture plate with a cell density of 10 5 / hole.
[0030] ② Take the rAAV gene drug and add it to the cell culture plate in step ① after appropriate dilution. The amount of rAAV gene drug added to each well is 10 6 Genome titer (vg).
[0031] ③The transduction titer of rAAV gene drug rAAV / Fluc was measured using the luciferase reporter gene detection kit produced by Beyontian Factory, and calculated based on the expression level (pg) of Fluc in each well.
Embodiment 3
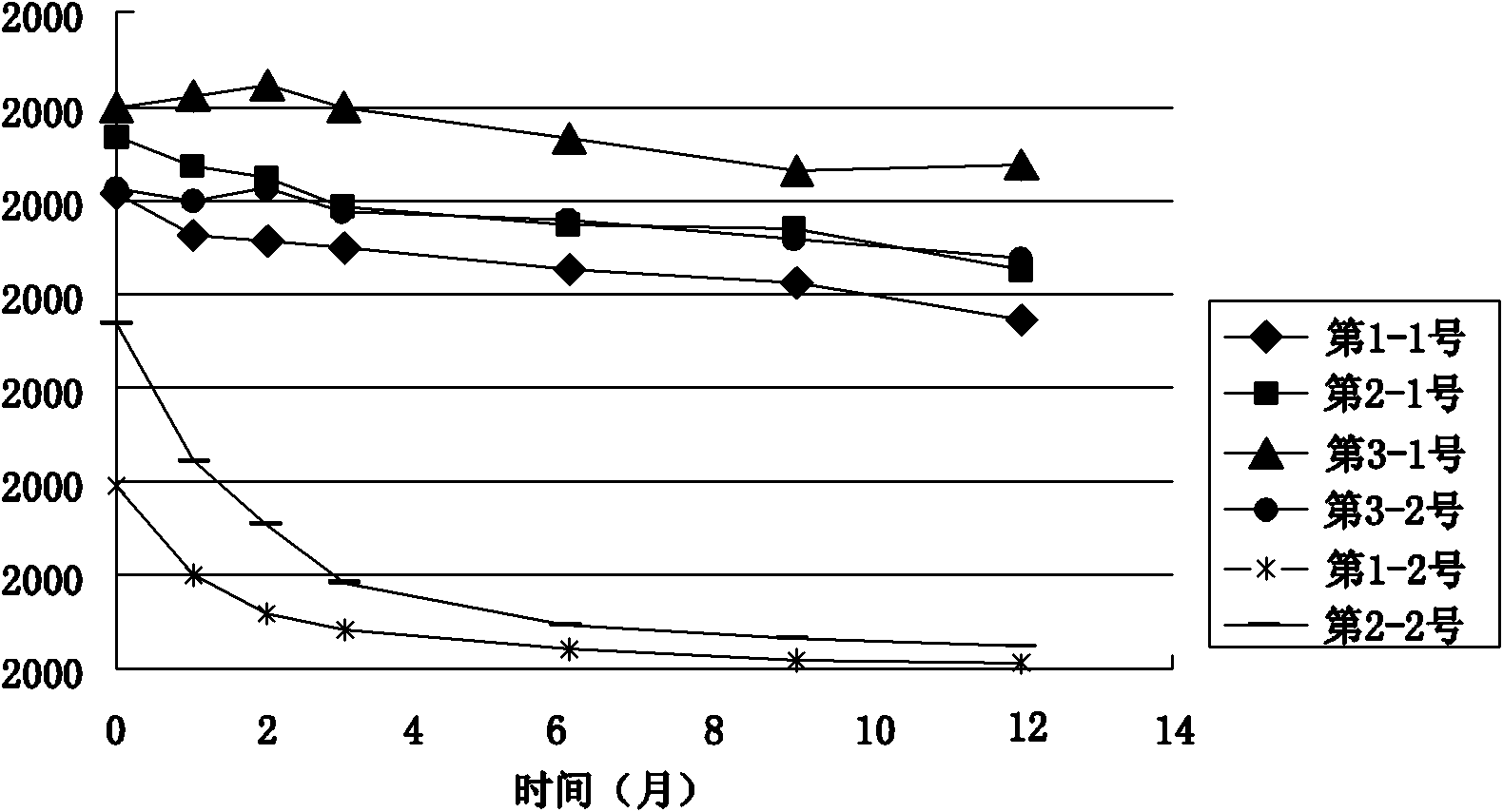
[0033] The stock solution of the rAAV gene drug prepared in Example 1 was taken, and the solvent was replaced by ultrafiltration to obtain the preparations in Table 1 respectively. PBS buffer (pH 7.4) commonly used in the laboratory was used as the control solvent. Table 1 is the preparation table of rAAV gene drug stability research preparation, wherein the preparation of PBS buffer solution: Na 2 HPO 4 12H 2 O 3.868 mg, NaH 2 PO 4 2H 2 O 0.395mg, NaCl 6.8mg, dissolved in 1ml of water for injection. Of course, the amount of adding excipient components in the prescription can be any value in the following range and can realize the purpose of the present invention: add human serum albumin 0.5-5mg, sodium citrate 10-50mg, citrate in every 1ml water for injection Citric acid 0.1-1.0mg, polyether F-68 0.001-0.1mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com