New application of protein Arginine methyltransferase 5
An arginine methyl and protein technology, applied to cells modified by introducing foreign genetic material, using a vector to introduce foreign genetic material, fermentation, etc., can solve the problem of less CHO cell lines, cell apoptosis, and foreign protein expression levels. limited issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1, Construction of a CHO-K1 cell line stably expressing PRMT5
[0024] 1. Construction of recombinant plasmid pCMV-Flag-PRMT5
[0025] 1. Using the plasmid (Incyte Full Length Human cDNA Clone; Open Biosystems plasmid product, catalog number IHS1380-97430767) containing the full-length sequence of PRMT5cDNA as a template, PCR amplification was carried out to obtain the PCR amplification product (containing the full-length fragment of the PRMT5cDNA sequence , see sequence 2) of the sequence listing.
[0026] The primer pairs for PCR amplification are as follows:
[0027] Upstream primer: 5'-G GAATTC GGCACGAGGGCGAGGAGAAAGATGGCGGCGATGGCG-3' (EcoRI);
[0028] Downstream primer: 5'-CCG CTCGAG CTAGAGGCCAATGGTATATGAGCG-3' (XhoI).
[0029] The annealing temperature for PCR amplification was 56°C.
[0030] 2. Digest the PCR amplification product with restriction endonucleases EcoRI (TAKARA, catalog number D1010A) and XhoI (TAKARA, catalog number D1094A), and reco...
Embodiment 2
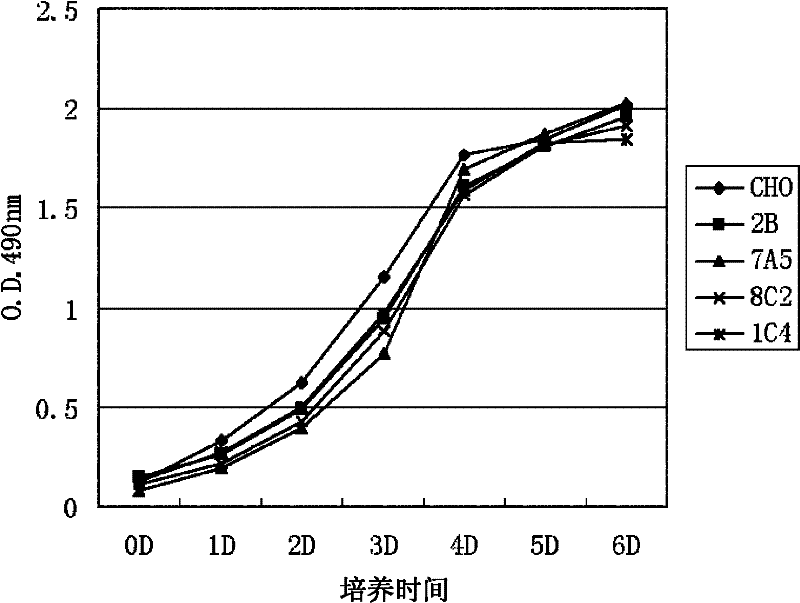
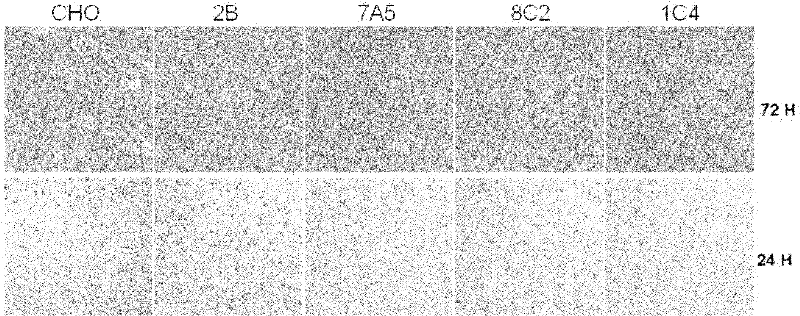
[0045] Embodiment 2, the proliferation situation of CHO-PRMT5
[0046] The same number of cells (5~8×10 4 ), CHO-K1 cells (CHO), CHO-2B cells (2B) and three different CHO-PRMT5 cell lines (7A5, 8C2 and 1C4) were evenly spread in Φ60mm plates, and medium A (containing 5 % volume percent calf serum) and medium B (containing 0.5% volume percent calf serum) for culture. Three replicate dishes were set up for each culture treatment, and the results were statistically averaged. 24hr, 48hr and 72hr after plate plating were taken and the number of cells was counted. Cell growth was quantitatively analyzed hourly by the MTT assay.
[0047] The results of MTT analysis when medium A is used are shown in figure 2 . For photos of cells using medium B, see image 3 . In culture medium A (5% calf serum), there was no significant difference in cell expansion observed under a microscope, and the quantitative analysis results of MTT method also showed that the cell growth rate was almos...
Embodiment 3
[0048] Example 3, application of CHO-PRMT5 cell line to express IL2 protein
[0049] 1. Preparation of recombinant plasmid IL2-pEGFP-N3
[0050] 1. PCR amplification of the insert
[0051] Using the plasmid containing the full-length sequence of IL2 cDNA (Incyte Full Length Human cDNA Clone; Open Biosystems plasmid product, catalog number IHS1380-97431972) as a template, perform PCR amplification to obtain PCR amplification products (containing The full-length fragment of the IL2 cDNA sequence; the DNA shown in the 1st to 459th nucleotides from the 5' end of the sequence 4 of the sequence listing).
[0052] The primer pairs for PCR amplification are as follows:
[0053] Upstream primer: 5'-CCC AAG CTT GCC ACC ATG TAC AGG ATG CAA CTC C-3';
[0054] Downstream primer: 5'-CG GA ATT C CA AGT CAG TGTTGAGATGATGC-3'.
[0055] 2. Digest the PCR amplification product of step 2 with restriction endonucleases HindIII and EcoRI, and recover the target fragment of about 470bp.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com