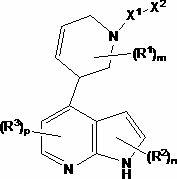
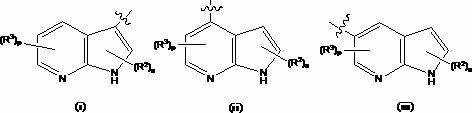
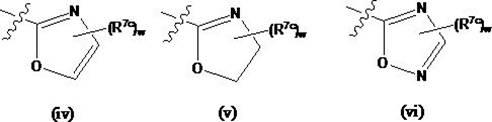
Compounds useful as inhibitors of protein kinases
A technology of compounds, solvates, in the field of compounds useful as protein kinase inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0335] N-[(1S)-2-Hydroxy-1-phenylethyl]-4-(1H-pyrrolo[2,3-b]pyridin-3-yl)-3,6-dihydropyridine-1(2H )-formamide
Embodiment 1A
[0337] (S)-2-(tert-butyldimethylsilyloxy)-1-phenylethylamine (ethanamine)
[0338] (S)-2-amino-2-phenylethanol (1.04g, 7.61mmol), tert-butylchlorodimethylsilane (1.15g, 7.62mmol), triethylamine (2.15ml, 15.4mmol), N , N-dimethylpyridin-4-amine (23mg, 0.19mmol) / dichloromethane solution was stirred overnight at room temperature, quenched with saturated aqueous NaHCO3, extracted with dichloromethane, dried (Na 2 SO 4 ), filtered, and concentrated to give 1.85 g of a clear oil, which was used without purification.
Embodiment 1B
[0340] (S)-N-(2-(tert-butyldimethylsilyloxy)-1-phenethyl)-4-(1H-pyrrolo[2,3-b]pyridin-3-yl)- 5,6-Dihydropyridine-1(2H)-carboxamide
[0341] A solution of the product of Example 1A (127mg, 0.505mmol), triphosgene (52.1mg, 0.176mmol), and triethylamine (0.25ml, 1.8mmol) / dichloromethane (2ml) was stirred at room temperature for 2 hours. 3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-pyrrolo[2,3-b]pyridine (0.10 g, 0.50 mmol) was added and stirred at room temperature for 2 hours. N,N-Dimethylformamide (1 ml) was added to dissolve, and the mixture was stirred overnight, diluted with ethyl acetate, washed with water and brine, dried (Na 2 SO 4 ), filtered, concentrated and chromatographed (3% methanol / dichloromethane) to give the product as a clear gum (0.186 g, 0.391 mmol).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com