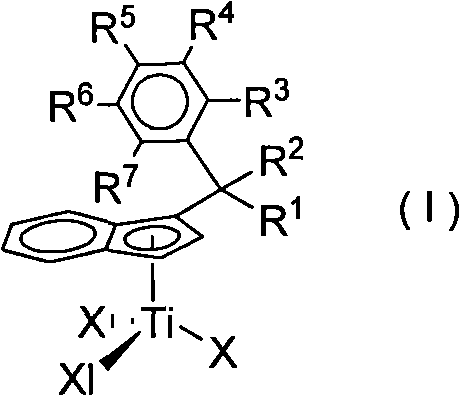
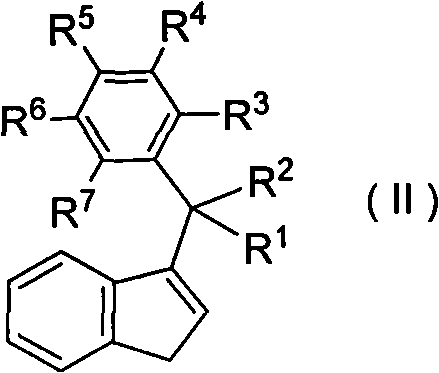
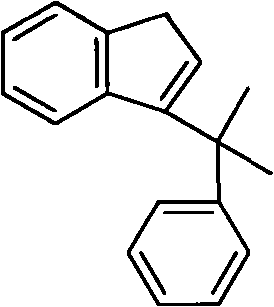
High-activity and high-selectivity ethylene trimerization catalyst as well as preparation method and application thereof
A high-selectivity, ethylene trimerization technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of low activity and achieve good catalytic effect , the effect of reducing the cost of industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of Substituted Indene Titanium Complex C1
[0035] (1) Preparation of Ligand Compound L1
[0036] At -78°C, n-BuLi (2.4mol / L, 30mL) was added dropwise to a petroleum ether solution of bromobenzene (0.072mol), and reacted for 2-7 days. Filter, dissolve the solid with 60 mL of ether, add 6,6-dimethylbenzofulvene (6.065 g), react for 2 days, hydrolyze and purify to obtain 1.8 g of yellow viscous liquid with a yield of 20%. The structural formula is as follows:
[0037]
[0038] 1 H NMR (400MHz, CDCl3 ): δ7.35(d, J=7.4Hz, 1H), 7.27-7.22(m, 2H), 7.20-7.13(m, 2H), 7.12-7.05(m, 1H), 6.99(td, J=7.4 Hz, J=0.7Hz, 1H), 6.91(t, J=7.4Hz, 1H), 6.63(d, J=7.7Hz, 1H), 6.40(t, J=2.1Hz, 1H), 3.33(d, J=2.1Hz, 2H), 1.59(s, 6H).
[0039] (2) Preparation of indene-titanium complex C1
[0040] Add n-BuLi (6mmol) dropwise to ligand L1 (1.40g, 0.006mol) in 50mL petroleum ether solution at 0°C, react overnight, then add TiCl at -78°C 4 (0.006mol), react for 12-24h. The solv...
Embodiment 2
[0046] Preparation of Substituted Indene Titanium Complex C2
[0047] (1) Preparation of Ligand Compound L2
[0048] At -78°C, add n-BuLi (2.4mol / L, 30mL) dropwise to a solution of bromobenzene (0.072mol) in petroleum ether, react for 2-7 days, filter, dissolve in ether, add 6,6- 8.326 g of pentylbenzofulvene was reacted for 2 days, hydrolyzed and purified to obtain 5.4 g of a light yellow solid with a yield of 46%. The structural formula is as follows:
[0049]
[0050] 1 H NMR (400MHz, CDCl 3 ): δ7.45-7.38(m, 3H), 7.29-7.23(m, 2H), 7.13(t, J=7.3Hz, 1H), 7.09-6.97(m, 3H), 6.52(t, J=2.1 Hz, 1H), 3.41(d, J=2.1Hz, 2H), 2.40-2.32(m, 2H), 2.26-2.13(m, 2H), 1.71-1.56(m, 5H), 1.53-1.39(m, 1H).
[0051] (2) Preparation of complex C2
[0052] Add n-BuLi (6 mmol) dropwise to ligand L2 (1.65 g, 0.006 mol) in 50 mL of n-hexane solution at 0°C, react overnight, then add TiCl at -78°C 4 (0.006mol), react for 12-24h. The solvent was removed, washed with n-hexane, filtered, and t...
Embodiment 3
[0058] Preparation of Substituted Indene Titanium Complex C3
[0059] (1) Preparation of Ligand Compound L3
[0060] At -78°C, add n-BuLi (2.4mol / L, 30mL) dropwise to a petroleum ether solution of p-methylbromobenzene (0.072mol), react for 2-7 days, filter, dissolve with ether, add 6, 6.177 g of 6-dimethylbenzofulvene was reacted for 2 days, hydrolyzed and purified to obtain 2.3 g of a light yellow viscous liquid with a yield of 23%. The structural formula is as follows:
[0061]
[0062] 1 H NMR (400MHz, CDCl 3 ): δ7.49(d, J=7.3Hz, 1H), 7.31-7.23(m, 2H), 7.18-7.10(m, 3H), 7.06(t, J=7.4Hz, 1H), 6.81(d, J=7.6Hz, 1H), 6.52(s, 1H), 3.46(s, 2H), 2.36(s, 3H,), 1.73(s, 6H).
[0063] (2) Preparation of complex C3
[0064] Add n-BuLi (6mmol) dropwise to ligand L3 (1.49g, 0.006mol) in 50mL petroleum ether solution at 0°C, react overnight, then add TiCl at -78°C 4 (0.006mol), react for 12-24h. The solvent was removed, petroleum ether was added to wash, and the filtrate was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com