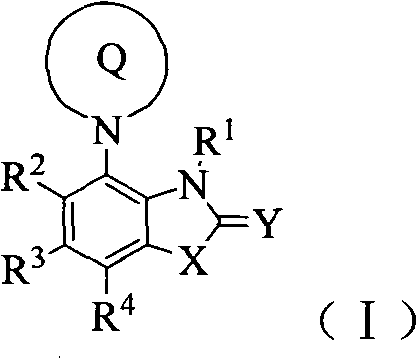
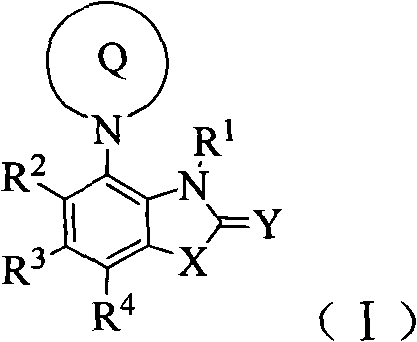
Benzocyclodirivative
A compound and alkyl technology, applied in the field of medicine, can solve the problems of not meeting clinical needs and limited varieties of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Example 1 (R)-2-[[7-(3-aminopiperidin-1-yl)-3,3-dimethyl-2-oxoindolin-1-yl]methyl]benzene formyl nitrile Compound 1) preparation of hydrochloride
[0193]
[0194] (1) Preparation of 1,3-dibromo-2-nitrosobenzene
[0195]
[0196] 9.8g (39mmol) of 2,6-dibromoaniline and 30g (139mmol) of 80% m-chloroperoxybenzoic acid were added to 500mL of dichloromethane, and reacted at room temperature for 3 hours to obtain 7.24g of the product, yield: 70.0 %.
[0197] (2) Preparation of 1,3-dibromo-2-nitrobenzene
[0198]
[0199] At room temperature, 1.23 g (4.64 mmol) of 1,3-dibromo-2-nitrosobenzene was dissolved in glacial acetic acid (25 mL) and H 2 o 2 (25mL), add HNO 3 (0.83mL), heated to 90°C, reacted for 2h, added 50mL of water, filtered to obtain 0.67g of product, yield: 51.4%.
[0200] (3) Preparation of (R)-1-(3-bromo-2-nitrophenyl)piperidin-3-ylcarbamate tert-butyl ester
[0201]
[0202] Dissolve 1,3-dibromo-2-nitrobenzene 8.4g (30.0mmol), (R)-piperi...
Embodiment 2
[0220] Example 2 (R)-2-[[7'-(3-aminopiperidin-1-yl)-2'-oxospiro[cyclopropane-1,3'-indoline]-1'- base]methyl]benzene Preparation of forminonitrile (compound 2) hydrochloride
[0221]
[0222] (1) Preparation of 2-(3-fluoro-2-nitrophenyl) diethyl malonate
[0223]
[0224] Add K 2 CO 3 26.7g (0.193mol), stirred and reacted overnight at 60°C. After the reaction was completed, it was cooled to room temperature, and ice water was added. The reaction mixture was extracted with ethyl acetate (3×50 mL), the combined organic layers were washed with brine (2×100 mL), MgSO 4 After drying, filtering and concentrating in vacuo, 26.8 g of a light yellow oily product was obtained, with a yield of 83.8%, which was directly used in the next reaction without further purification.
[0225] (2) Preparation of 2-(3-fluoro-2-nitrophenyl)acetic acid
[0226]
[0227] A mixture of 26.8 g (0.896 mol) of diethyl 2-(3-fluoro-2-nitrophenyl) malonate and 200 mL (6N) of HCl was refluxed...
Embodiment 3
[0254] Example 3 (R)-2-[[7-(3-aminopiperidin-1-yl)-3-methyl-2-oxo-2,3-dioxo-1H-benzo[d]imidazole -1-yl] A The preparation of base] benzonitrile (compound 5) trifluoroacetate
[0255]
[0256] (1) Preparation of (R)-1-(3-fluoro-2-nitrophenyl)piperidin-3-ylcarbamate tert-butyl ester
[0257]
[0258] Add 6 mL of ethanol, 0.21 mL (2.0 mmol) of 1,3-difluoro-2-nitrobenzene, 480 mg (2.4 mmol) of tert-butyl (R)-piperidin-3-ylcarbamate into the dry reaction flask, 0.5 mL of triethylamine was heated to 100° C. and stirred for 12 hours, the reaction solution was decompressed, and a yellow liquid was precipitated to obtain 0.7 g of crude product, which was directly used in the next reaction.
[0259] (2) Preparation of (R)-1-[3-(methylamino)-2-nitrophenyl]piperidin-3-ylcarbamate tert-butyl ester
[0260]
[0261] In the dry reaction bottle, add (R)-1-(3-fluoro-2-nitrophenyl)piperidin-3-ylcarbamate tert-butyl 0.7g (2.1mmol), dissolve in 8mL butanol, Add 6 mL of 27% methyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com