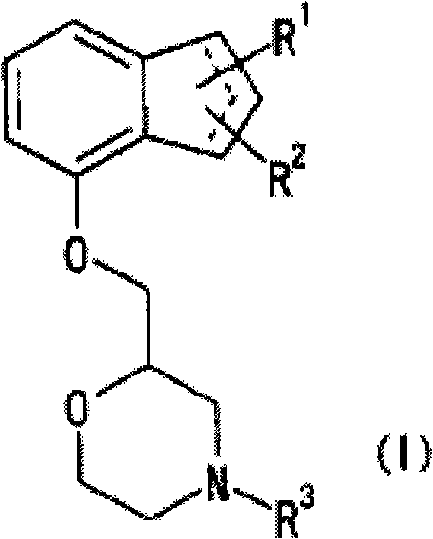
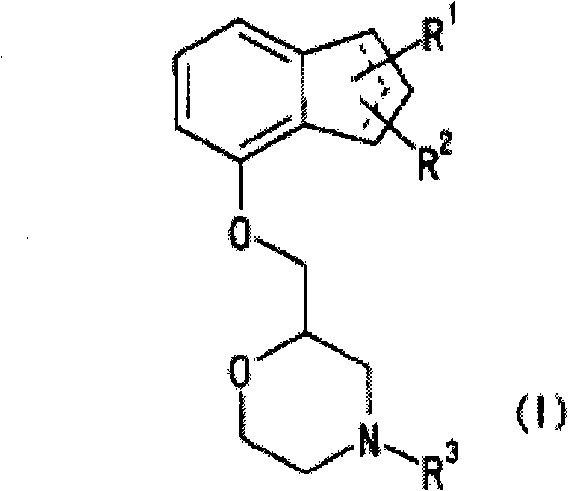
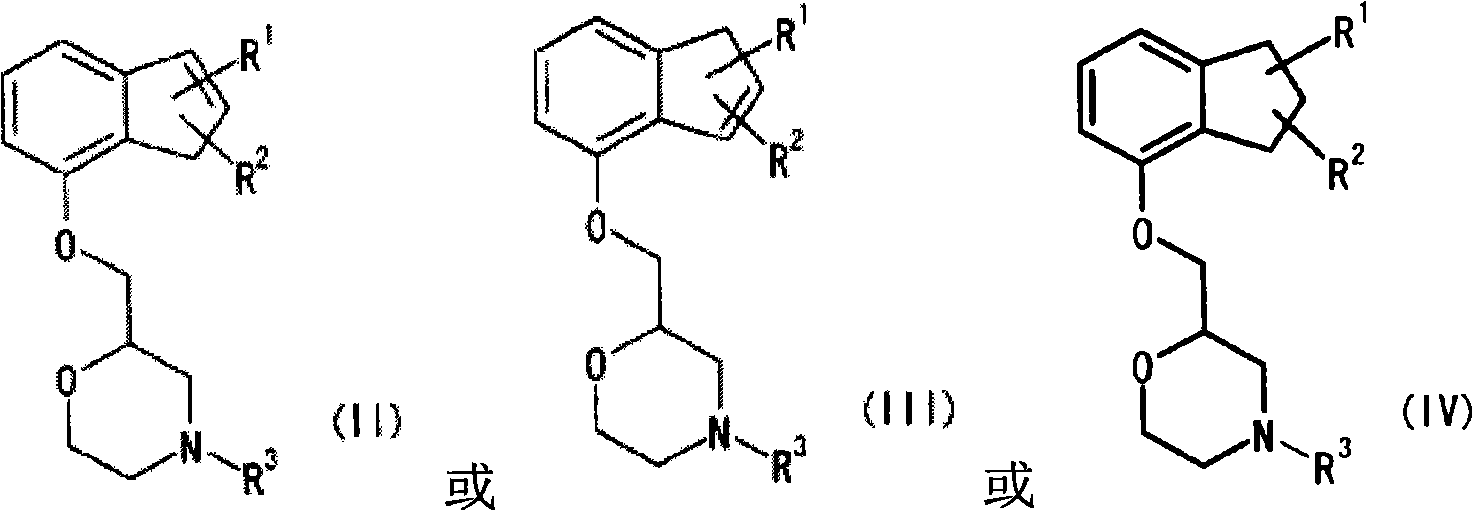
Novel pharmaceutical composition for treating nociceptive pain
A kind of sensory pain, composition technology, applied in the field of new pharmaceutical composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Synthesis of (+)-2-[(inden-7-yloxy)methyl]morpholine (hereinafter referred to as compound A) hydrobromide
[0084]Under ice cooling and stirring, 2.92 g of 47% hydrobromic acid was dissolved in a mixed solvent of 500 ml of water-500 ml of methanol. Under the same conditions, 1000 ml of diethyl ether was added, followed by 10 g of Compound A(-)-(2R,3R)-di-O-benzoyl tartrate, followed by vigorous stirring for 30 minutes. After standing still, the water layer was separated and washed 4 times with 500ml diethyl ether. The resulting aqueous layer was concentrated under reduced pressure at room temperature or lower, dissolved in methanol, and filtered. After the filtrate was concentrated under reduced pressure at a temperature below room temperature, it was azeotroped with toluene at a temperature below room temperature. After the obtained oily substance was crystallized from isopropanol-diethyl ether, the crystals were filtered off. The obtained crystals were recrystalliz...
Embodiment 2
[0089] The preparation of embodiment 2 compound A besylate (α type crystal)
[0090] Under ice cooling, add 1000mg of compound A(-)-(2R,3R)-di-O-benzoyl tartrate into 20ml ethyl acetate-20ml saturated aqueous sodium bicarbonate solution, and stir rapidly under the same conditions After that, the organic layer was separated. After the obtained organic layer was washed 2 times with 20 ml of ice-cooled saturated aqueous sodium bicarbonate solution, then with 10 ml of ice-cooled saturated brine, and after drying with anhydrous magnesium sulfate, it was added to the , 269mg of benzenesulfonic acid in 10ml of ethanol formed in the solution. After concentrating the resulting solution under reduced pressure at a temperature below room temperature, crystallization was carried out with ethanol-diethyl ether, and the crystals were filtered and washed with diethyl ether. The obtained crystals were recrystallized from 3 ml of acetone-0.1 ml of water-3 ml of diethyl ether to obtain 422 mg...
Embodiment 3
[0096] Analgesic Effects of Monoiodoacetate (MIA)-Induced Osteoarthritis Model
[0097] This disease model was prepared according to the description in Toxicol Pathol 31(6), 619-624(2003). Male SD rats (6-7 weeks old, Chiya-ruzuriba, Japan) were anesthetized with halothane (Takeda Pharmaceuticals), and sodium iodoacetate (MIA; Sigma, St. Single injection (20mg / ml) into the joint cavity. MIA was dissolved in saline and 50 μl was injected with a 26G 0.5 inch needle. After 3 weeks of administration of MIA (after the occurrence of osteoarthritis), the solvent (Vehicle) and the dilution obtained after diluting Compound A with a solvent to a predetermined concentration were orally administered to rats in each group (8 rats were used in each group). ). The solvent is distilled water. One hour after the administration, the weight difference between the left and right hind limbs was measured with an Incapacitance Tester (Linton Instrumentation, Norfork, UK). The inhibition rate of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com