Pharmaceutical composition with function of treating polycystic ovary syndrome
A technology of polycystic ovary and composition, which is applied in the field of pharmaceutical composition and its preparation with the effect of treating polycystic ovary syndrome, and can solve difficult problems such as radical cure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
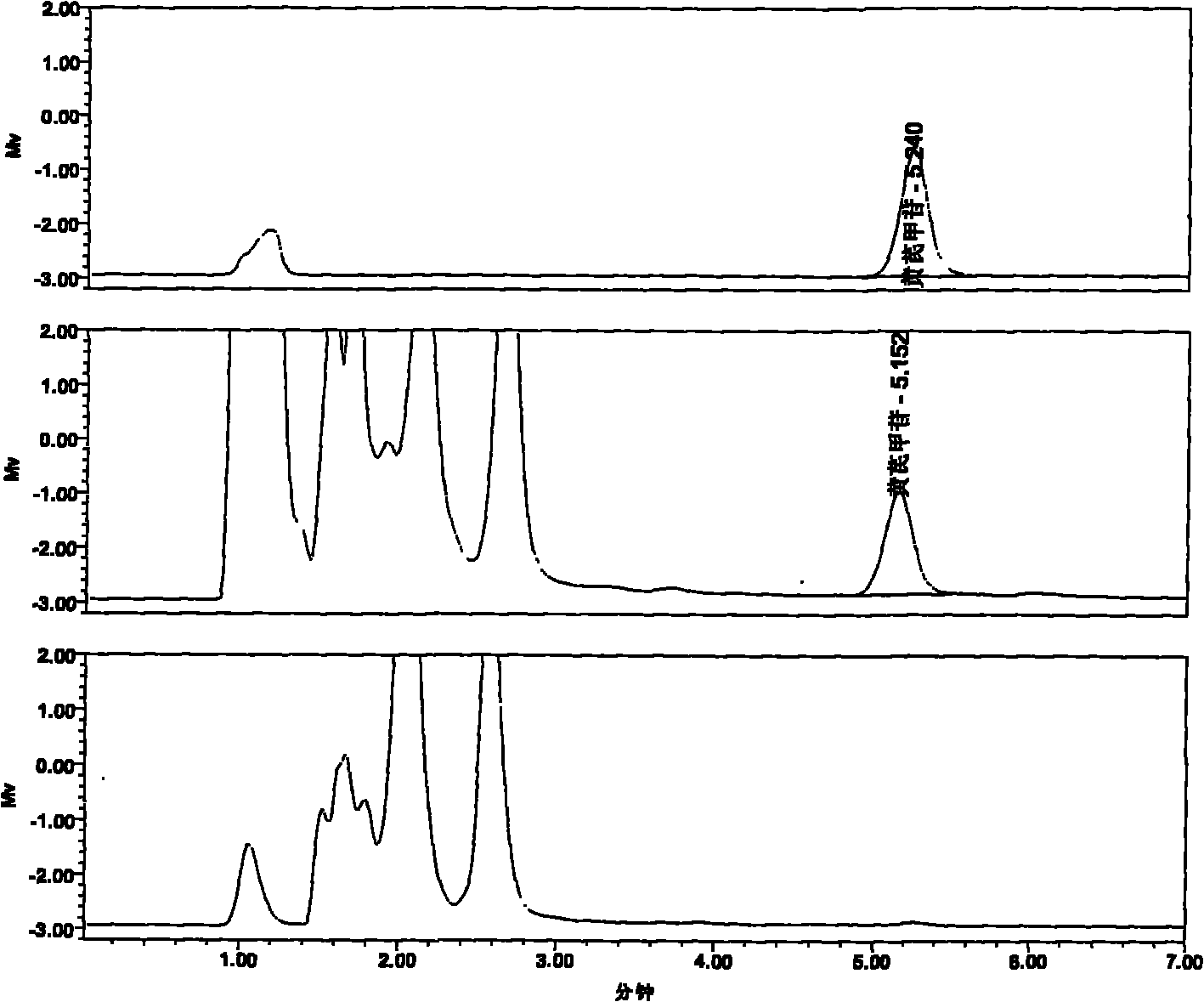
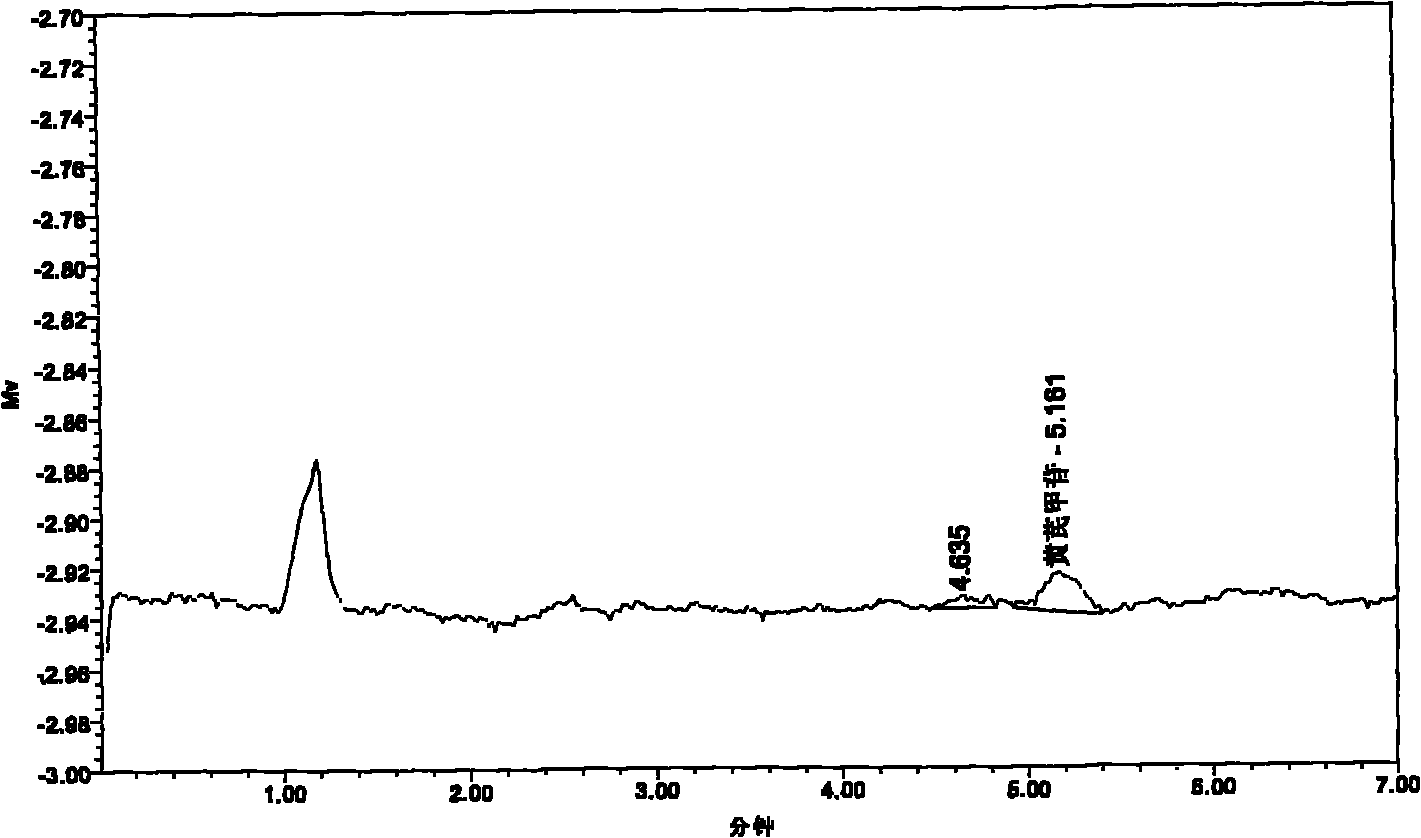
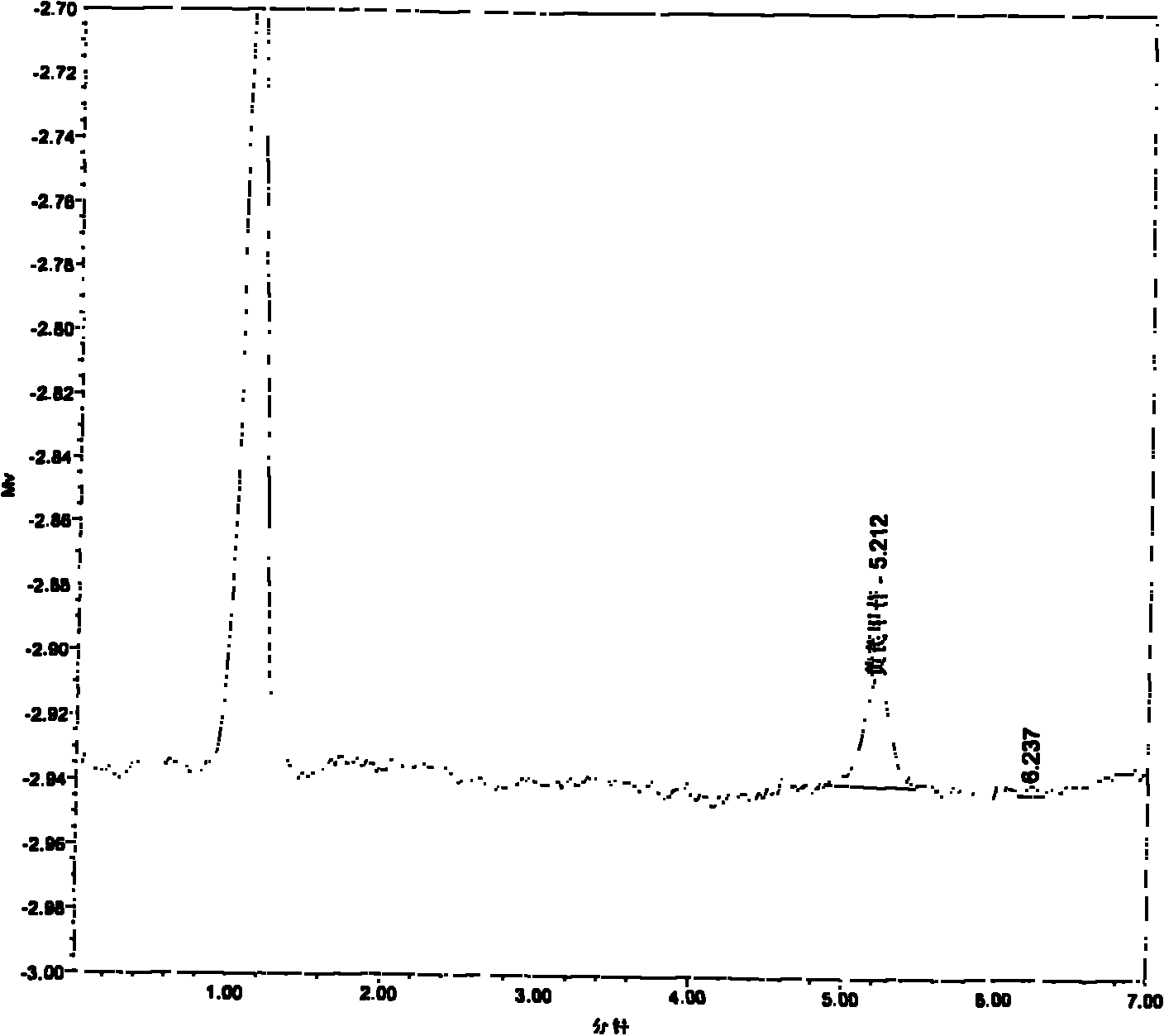
Image
Examples
Embodiment 1
[0315] Embodiment 1: pharmaceutical composition capsule of the present invention
[0316] Astragalus 500g Poria 300g Atractylodes 300g
[0317] Epimedium 300g Salvia 100g.
[0318] Take the above-mentioned raw materials, and extract Danshen with 6 weight times of 90% ethanol under reflux for 2 times, each time for 1 hour, combine the extracts, filter, recover ethanol from the filtrate, and set aside; the other four herbs of Astragalus, Poria, Epimedium and Atractylodes Add 8 times the weight of water to soak for 1 hour, decoct 2 times, 2 hours each time, filter, combine the filtrate, combine with the above standby liquid, concentrate into a thick paste with a density of 1.2-1.5 at room temperature, dry, and grind into fine powder , adding conventional auxiliary materials, made into 1000 capsules, each 0.35g, orally 2-3 times a day, 2-4 capsules each time.
Embodiment 2
[0319] Embodiment 2: pharmaceutical composition tablet of the present invention
[0320] Astragalus 700g Poria 150g Stir-fried Atractylodes 150g
[0321] Epimedium 450g Salvia 150g.
[0322] Get above-mentioned raw material, add conventional adjuvant, make pharmaceutical composition tablet of the present invention according to conventional process.
Embodiment 3
[0323] Embodiment 3: pharmaceutical composition granule of the present invention
[0324] Astragalus 300g Poria 450g Atractylodes 450g
[0325] Epimedium 150g Salvia 80g.
[0326] Take the above-mentioned raw materials, and extract Danshen with 6 weight times of 90% ethanol under reflux for 2 times, each time for 1 hour, combine the extracts, filter, recover ethanol from the filtrate, and set aside; the other four herbs of Astragalus, Poria, Epimedium and Atractylodes Add 8 times the weight of water to soak for 1 hour, decoct 2 times, 2 hours each time, filter, combine the filtrate, combine with the above standby liquid, concentrate into a thick paste with a density of 1.2-1.5 at room temperature, dry, and grind into fine powder , add conventional auxiliary materials, and prepare the pharmaceutical composition granules of the present invention according to conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com