Nutritional composition for improving muscle function and daily activity
A nutritional composition and functional technology, applied in the field of compositions for improving daily activities of mammals and compositions for improving muscle function of mammals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
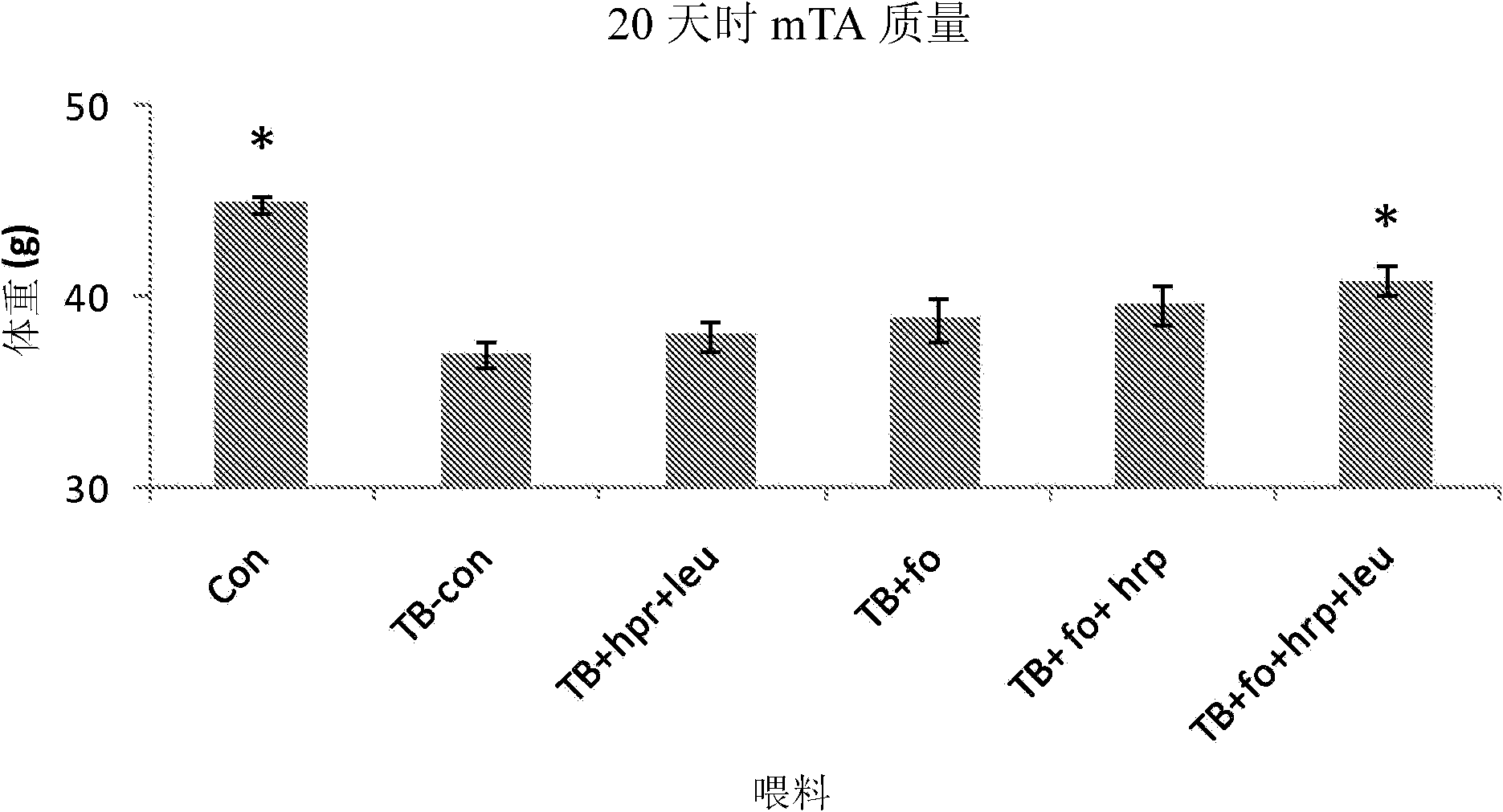
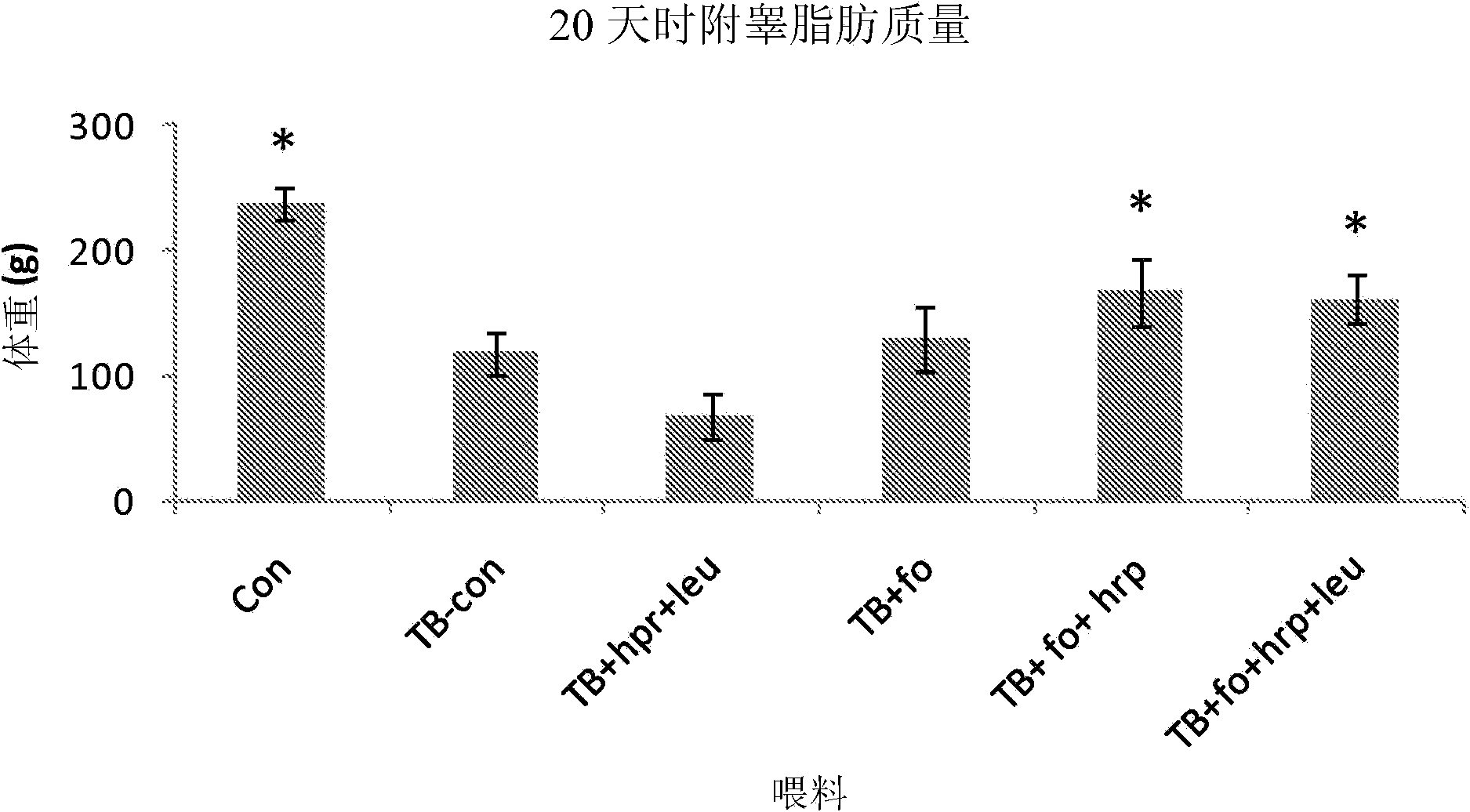
[0170] Materials and Method
[0171] Animals. In a temperature-controlled place (12:12 dark-light cycle, where the constant room temperature is 21±1℃), male CD2F1 mice (BALB / cx DBA / 2, Harlan / Charles River the Netherlands). After one week of acclimatization, the mice were divided into weight-matched groups: (1) a control group receiving control food, (2) a tumor-bearing group receiving control food, and (3) a tumor-bearing group receiving experimental food. The data given is derived from a combination of several experimental experiments that have the same animal characteristics and experimental procedures (unless otherwise stated) and differ only in the experimental food used. All experimental procedures were approved by the Animal Ethical Committee (DEC consult, Bilthoven, The Netherlands) and followed the principles of good laboratory animal care.
[0172] Experimental food (type A and B experiments). The experiment is divided into: (A) Designed to test the nutritional componen...
example 2
[0205] Example 2: Formulation example
[0206] The sip feed may specifically contain macronutrients in the range specified in Table 3. Specific examples are given in Table 4. In addition, one or more micronutrients (such as minerals, vitamins, etc.) and / or one or more other food-grade additives (such as flavoring agents, preservatives, non-protein amino acids, such as carnitine) may be present.
[0207] Table 3: Nutritional composition of sip feed (per 100ml)
[0208] Protein substances (equivalent) (g)
9-12
-Contains total whey protein
1-9
-Contains total leucine (g)
1.5-2.5
-Leucine is a free amino acid (g)
0.9-1.5
Carbohydrate (g)
10-25
Fat (g)
2-6
2-6
0.8-2
-Among them EPA, DHA, ETA, DPA
0.2-2
Soluble fat, food g
1-4
[0209] Table 4: Nutritional composition of sipped feed (per 100ml)
[0210] Protein substances (equivalent) (g)
10.1
-Contains total whey protein
2.9
-Contains total leucine (g...
example 3
[0220] Example 3: Muscle protein synthesis in colorectal cancer patients after nutritional supplementation
[0221] The ability of sipped feeds from possible compositions containing specific nutrient combinations as described in Table 4 to affect the rate of muscle protein synthesis was tested and compared with the control products described in Table 8.
[0222] Table 8. Supplement composition
[0223]
[0224] *SNC: 2.9g whey protein, 1.1g free leucine, balanced casein, control casein only
[0225] Research design and methodology
[0226] Research subjects. Subjects are registered based on the inclusion / exclusion criteria described below. All subjects were able to walk, sit and stand independently. Prior to this study, screening procedures were not performed in the context of cancer care. A total of 24 subjects (12 in each group) completed the program. The criteria for inclusion are as follows: (1) imaging evidence of cancer, (2) age> 40 years (male and female), (3) ability to sign...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com