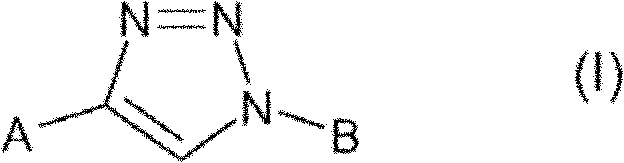Triazole derivatives and their use as nicotinic acetylcholine receptor modulators
A technology of triazole derivatives and mixtures, which is applied in the field of new triazole derivatives, and can solve problems such as complex understanding of physiological functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] 3-Trimethylsilylethynyl-benzonitrile (intermediate compound)
[0144]
[0145] Tetrahydrofuran (50 mL) was added to 3-bromobenzonitrile (2 g, 10.9 mmol), triphenylphosphine (0.3 g, 1.1 mmol), cuprous iodide (0.21 g, 1.1 mmol) and (bistriphenylphosphine) In a mixture of palladium(II) dichloride, the mixture was degassed. Triethylamine (3.0 mL, 21.8 mmol) was added and the mixture was stirred at room temperature under nitrogen for 10 minutes. After 15 minutes. Trimethylsilylacetylene (1.4 g, 14.1 mmol) was added dropwise. The reaction mixture was stirred overnight at room temperature.
[0146] No ingredients left. The mixture was concentrated under reduced pressure, hexane (50 mL) was added to the residue and filtered. The filtrate was washed with water and evaporated to a brown solid. Yield 1.9 g (89%).
[0147] 3-Ethynyl-benzonitrile (intermediate compound)
[0148]
[0149] Potassium carbonate (1.3 g, 9.4 mmol) was added to a solution of 3-trimethylsilyle...
Embodiment 2
[0198] 5-(4-Pyridin-3-yl-[1,2,3]triazol-1-yl)-furan-2-carboxylic acid (intermediate compound)
[0199] Potassium hydroxide (0.15 g, 2.8 mmol) was added to methyl 5-(4-pyridin-3-yl-[1,2,3]triazol-1-yl)-furan-2-carboxylate (0.75 g, 2.8 mmol) in tetrahydrofuran / water (1 / 1) at 0°C. The reaction mixture was stirred at room temperature for 2 hours. Hydrochloric acid (1.5N) was added until the pH was between 5 and 6 and the solid form was isolated by filtration. The white solid was dried in vacuo. Yield 0.66 g (92%).
[0200] 5-(4-Pyridin-3-yl-[1,2,3]triazol-1-yl)-furan-2-carboxylic acid amide (intermediate compound)
[0201]
[0202] 5-(4-Pyridin-3-yl-[1,2,3]triazol-1-yl)-furan-2-carboxylic acid (0.65 g, 2.5 mmol) was added to oxalyl chloride (10 mL, 118 mmol) , N,N-Dimethylformamide (20 μL, 0.25 mmol) was added to the mixture. The reaction mixture was stirred overnight at 45 °C.
[0203] The reaction mixture was concentrated to remove oxalyl chloride, and the residue wa...
Embodiment 3
[0209] biological activity
[0210] Characterization of hα4β2 positive allosteric modulators using FLIPR
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com